Herbal contraceptive effect of Abrus precatorius, Ricinus communis, and Syzygium aromaticum on anatomy of the testis of male Swiss albino mice
Abstract
The present research was designed to investigate in vivo herbal contraceptive effects on the anatomy of the testis in male Swiss albino mice (Mus musculus) and to observe that whether the herbal extracts depart any baleful effects on the genitalia or not. Swiss albino mice at age of 30 days were divided into two groups: control and treated group. From day 60, the treated group was administered orally with an aqueous extract (4.4 mg/kg body weight) prepared from the herbal plants. At day 97 (after treatment for 6 weeks) both the control and treated mice were sacrificed. Testes were collected for anatomical (gross and histomorphological) studies. Hematoxylin and Eosin staining was performed for the histo-morphological studies. In the present study, the gross anatomy of the testis were significantly (p<0.05) reduced and pale in color in treated mice in comparison to the control group. Histologically, number of seminiferous tubules, sertoli & leydig cells and amount of spermatozoa within the lumen of seminiferous tubules were decreased. Derangement of the seminiferous tubules along with the presence of a thick fibrous layer and vacuoles were found within and between the seminiferous tubules in the treated group. The aforesaid changes with a good contraception rate (75%) and devoid of any baleful effects even on the female were found, where the contraceptive pills are known to have many side effects. Considering above all, the mixture of plants seems to be effectively worked on the male gonad (testis) suggesting that the herbal extracts might be future use-worthy.
INTRODUCTION
Contraception directly affects the population size, a crucial issue for human communities as well as for the well-being of the women, as the available pills are mostly for the women which have negative impact on the different organs of human body. It would be even better if we could obtain figures on the pregnancy rate, for that would give us a better estimate of reproduction control and help us to distinguish contraception from the abortion and infanticide. Birth control was widely practiced in the pre-agricultural and nomadic societies also [1]. Early oral contraceptives were initially approved for the indication of “menstrual regulation”, not contraception. Several dietary and herbal supplements can interfere with the efficacy of birth control pills [2]. Herbal products can be used both in case of male and female so the method of contraception will be more effective. Some plants, flowers and seeds can also be used for this purpose [3]. There are also many herbs that are very innocuous, which can be used by virtually human being without any baleful effects on the health [4, 5]. It is eternal that nature makes our lives possible at every aspect as it is the chief depot of all the primary resources for human medicines [6]. People are becoming more dependent on the herbal medicines rather than the synthetic or chemical drugs as the herbs are usually free from any side effects [7]. Herbal extracts/products have been used for the medicinal values from the ancient time having a long history [8]. China and India are the two countries that have done quite a bit of researches on herbal contraceptives. Thus an attempt has been made to review plants which have been used as herbal contraceptives [9].
The Abrus precatorius commonly known as rosary pea, was formerly used to weigh gems and precious stones. As per factual recordings the Abrus precatorius plant was used to weigh the famous Kohinoor diamond as well. In China the herb of Abrus precatorius is used as a folk-medicine for the treatment of bronchitis, aryngitis and hepatitis. Because of their platelet inhibiting activity, abrus and quinones present in the Abrus precatorius are supposed to be the active substances [10].
The seeds of Abrus precatorius yield alkaloids, a fixed oil, steroids, lectin, flavonoids, and anthocyanins. The alkaloids of the seeds are abrin, hypaphorine, choline and precatorine. The oil content of seed is only 2.5%, which is rich in oleic acid and linoleic acids. α-sitosterol, stigmasterol, 5α-cholanic acid, abricin, and cholesterol are the steroids present. The colour of the seed is due to glycosides of abranin, pelargonidin, cyaniding, and delphinidin [11]. Abrin, abricin have the antifertility effect on the testis.
In ancient times, farmers knew to keep their livestock away from the Ricinus communis or castor bean as it is highly toxic if the dose is not maintained properly there is the chance to lose it [12]. The seeds have been also used in folk medicine against a wide variety of diseases [13]. The use of these proteins in medical treatment since ancient times is reviewed.
A sapogenol, abrisapogenol J, sophoradiol, its 22-O-acetate, hederagenin methyl ether, kaikasaponin III methyl ester, flavones such as abrectorin and aknone are the active constituents of the seeds. Lectins are the chief constituents of the seeds, the principal ones being abrin. Lectins are both toxic (abrin) and non toxic (abrus agglutinin) [14]. Abrin, saponins, sapongenols are considered to be used as the antifertility agent. Recently, these toxins have played important roles as experimental models to elucidate the intracellular trafficking of endocytosed proteins [15].
Syzygium aromaticum or cloves have been found in vessels dating as far back as 1721 BC. Native to the Molucca Islands, as many spices, cloves were once a treasured commodity prized by the ancient Romans [2]. The flowers are rich in kaempferol, quercetin, myricetin, isoquercetin (quercetin-3-glucoside), myricetin-3-L-arabinoside, quercetin-3-D-galactoside, dihydromyricetin, oleanolic acid, acetyl oleanolic acid, eugenol-triterpenoid A and eugenol-triterpenoid B [16].
Although they are underappreciated for their medicinal uses today, cloves have been used historically to treat many diseases. They have antiseptic, antibacterial, antifungal, antispasmodic, antiviral, antiparasitic, analgesic, and simulative properties making them a great overall healer. They can be used to stimulate the mind as well as prevent nausea & diarrhea; ease coughs, aid in digestion and even treats conditions like malaria and cholera. They can also be used topically to treat acne, sties (pimples) and sores [17].
Testis is the primary organ or gonad for the reproduction in case of male as it produces the spermatozoa (male gamete). In the previous studies of herbal contraception for male, gross anatomically, the size of the treated testes reduced and the weight also reduced comparing with the control [18] in the rabbit.
Normal male reproductive function is dependent on the normal functioning of the male reproductive organ and other accessory organs/structures. The male reproductive organ is the testis, which is primarily responsible for the production of spermatozoa. Sperm production occurs in the seminiferous tubules of the testis, which is controlled by testosterone, produced by the Leydig (interstitial) cells of the testis. Testosterone production is directly dependent on the concentration (or activity) of leutinizing hormone (LH), in the milieu secreted by the anterior pituitary gland. Follicle stimulating hormone (FSH), released also by the anterior pituitary stimulates the Sertoli cells of the testis which give support and nourishment to developing spermatozoa. The quality and quantity of spermatozoa produced will therefore depend on normalfunctioning of the testicular structures and reproductive hormones [19].
Whereas, some previous studies have mentioned that Oral administration of herbal extract over 4 weeks, caused significant (p≤0.05) effects on the serum levels of testosterone, LH and FSH. In our images we have also found that the number of leydig and sertoli cells are reduced as a result the production of LH, FSH and testosterone is hindered and cause reduction in the sperm production [20].
So we can say that with the chemical constituents act on the testis, reduce the hormonal level and cause the contraception.
Histologically, the treated seminiferous tubules showed reduction in number of the seminiferous tubules, mixing of spermatids of different stages of spermatogenesis within the lumen of the seminiferous tubules, intraepithelial vacuolation, loosening of germinal epithelium and occurrence of giant cells [21] in the Wister mice. In the lumen of the seminiferous tubules showed no matured spermatozoa but spermatids were present but the number reduced as well [22]. Outside of the seminiferous tubules leydig cells present and sertoli cells were present in between the spermatocytes within the seminiferous tubules. The reduction of the amount of the leydig and sertoli cells between and within the seminiferous tubules and their morphology also changed comparing with the control [23]. These changes in the anatomy of the testes were due to the treatment by the herbal extracts that was used for the purpose of contraception.
Therefore, the present research on the herbal (Abrus precatprius, Ricinus communis and Syzygium aromaticum) contraceptive effects on the anatomy of the testis and ovary in Swiss albino mice might be a frontier one.
MATERIALS AND METHODS
Experimental animals
Twenty Swiss albino mice (Mus musculus) male and female were divided into control group (C) and treated group (T), which were purchased at the age of 30 days (average body weight: 25-28 g) from the Animal Resource Center, International Centre for Diarrhoeal Disease Research, Bangladesh, Mohakhali, Dhaka. Before being used in the experiment, mice were reared for 15 days in order to be accustomed with the environment and also to reach the age of sexual maturity (since Swiss albino mice both male and female reach to their sexual maturity at 45-48 days). To observe the normal reproductive ability of the mice at the age of 45 days male and female mice were kept together and the first parity (first time delivery of offspring) was found at the age of 58 days (as the incubation period is 14 days). After that at the age of 60 days the experiment was started. The mice were housed in compartmentalized rectangular metallic cages (9x11x7 cube inches) wrapped with wire mesh and also in the deep bottom dishes to facilitate the sexual behavior. The mice had neither developmental disorders, detectable genital diseases nor other diseases that may cause any problem in the experiment or affect the result thereby.
Rearing and care
The mice were cared at Animal Care Room, Department of Anatomy and Histology, Faculty of Veterinary Science, Bangladesh Agricultural University in proper hygienic conditions, with experimental and normal feeding (standard pelleted feed for mice from ICDDR, B) ad libitum. During the experimental period, uniformity of the management practices was maintained. The ventilation of the rearing house of mice was sufficient as a standard one. The room temperature was 28±20C and relative humidity 70-80% with natural day and light. Before starting the experiment the mice were reared and observed for a normal cycle to clarify that whether they were reproductive or not. For this reason both the male and female were kept together and fed the normal mice pellet and water ad libitum for at least 15 days.
Experimental plant
The plants those were used for the purpose are: Abrus precatorious (Abrus), Ricinus communis (Castor bean) and Syzygium aromaticum (Clove). The seeds of the Abrus precatorius and Ricinus communis, fruit of Syzygium aromaticum were used in the extraction and to observe their effects on the testis
Extraction of the plant
The plant material (seed and fruit) was collected and air dried for 10 days under an open shade and pulverized with the help of a mortar and pestle to fine powder. Then 50 gm of powder was dissolved in 1000 ml (1 L) of distilled water in a conical flask. The mixture was intermittently shaken throughout the period of extraction using glass rod stirrer, but allowed to stand overnight and filtered with whatman No 1 filter paper into measuring cylinder and concentrated at 600C in an incubator and next stored in a refrigerator at 40C until used and modified by method described by Sagnuwan and Onyeyili (2010) [24].
Experimental treatment
Before starting the experiment the mice were fed for 15 days by normal feed (pellet) and water ad libitum and the regular record of the feed consumption and body weight gain were recorded to be accustomed with the environment. This recording was started at the 45th day of age of the mice. At the age of 60th day the treated groups were administered the aqueous extract of the herbal products (seed of Abrus precatorious, Ricinus communis and fruit of Syzygium aromaticum @ 4.4 mg/kg bwt.) orally for the purpose of birth control and on the previous day of treatment these groups were not given the 2nd meal. During the treatment the male and female were kept separately but after the treatment they were kept together. At the time of experiment the male and female were kept separated and after the treatment they were brought in contact to observe the efficacy of the extract. During the experimental period, the uniformity of the management practices was maintained.
Sample collection
Physiological data such as daily feeding status and body weight was taken on the 1st week, during the experiment and after end of the experiment. After 6 weeks of the experiment period, all the experimental animals of both the control and treated groups were sacrificed and the samples from the testis (left) were collected with the help of scalpel and forceps for the gross and histological observation. The average weight, length and diameter of the testes were measured (electrical weighing measure and scale). Then the collected samples were preserved in fixatives (10% formalin).
Tissue processing and staining
For the purpose of histological observation of the testis, 5 mm pieces were collected from testis and immersed in 10% formalin for 48 hours. Then, the sample was washed in 10% phosphate buffer solution for 3 hours, dehydration was done by passing the tissue in the ascending grade of alcohol, such as 70, 80, 90, 95, 100% (1), and 100% (2) each for 2 hour and finally 100% (3) for overnight, cleared in xylene and embedded in paraffin. Sections from the paraffin blocks were cut in 5 μm in thickness by using rotatory microtome. Then, the sections were stained with Meyer’s Hematoxylin and Eosin (H&E). The sections were protected by a thin cover slip attached to the slide with a mounting medium ‘DPX’. The samples were studied with the aid of light microscope.
Photography and illustration
Necessary photography was done during gross morphological and histological investigation for better illustration of the result. The gross anatomical pictures were taken directly from the organs by using digital camera and the histological pictures were taken from light microscope. The Olympus-BX-51 microscope was used and necessary illustration was carried out by Adobe Photoshop®.
Statistical analyses
During the study period we collected the data of daily feed intake and body weight weekly. After the study period we analyzed gross anatomical, histological and hematological data. Chi square test of all the collected data were then analyzed using IBM SPSS Statistics (version 21) software and revealed the results.
RESULTS
Gross Anatomy
Weight
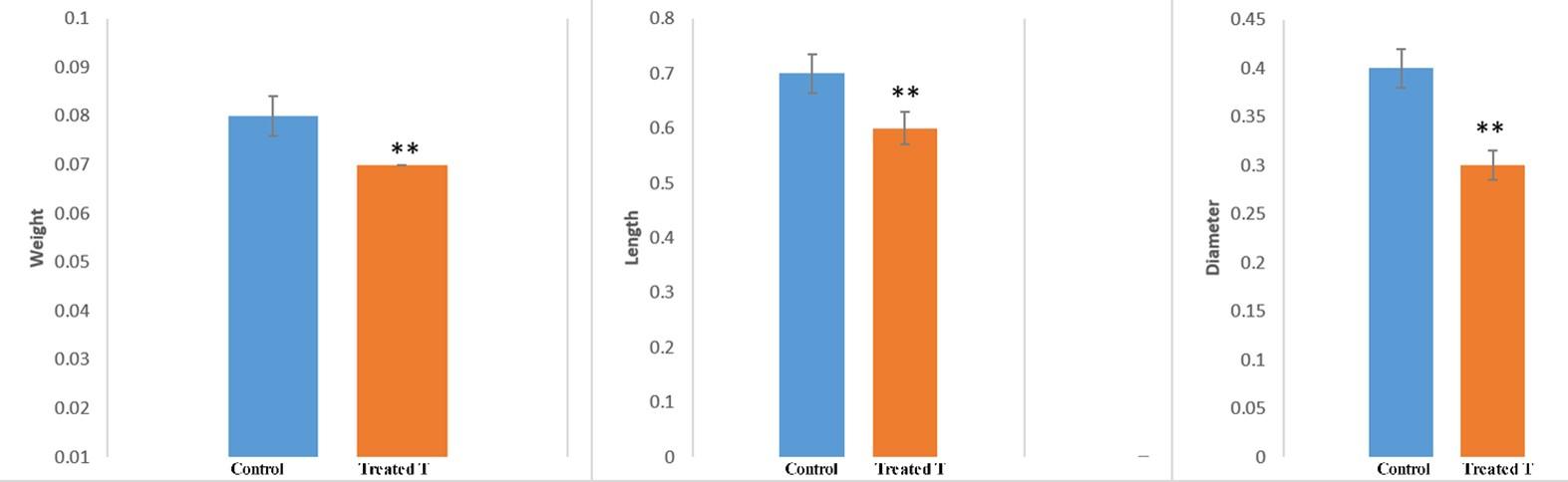
It was observed in the gross study that the mean weight of the testis was 0.08±0.02 gm in the control group (group C) and 0.07±0.00** gm in the treated mice (group T) (Figure 1), respectively. Results revealed that the weight of the testis significantly decreased (p<0.05) in the herbal extract treated mice of group T in comparison to the mice of the control group (C) (Figure 1).
Length
The changes in length of testis during the experimental period in different groups including the control were given in Figure 1. It was found in the gross study that the mean length of the testis was 0.70±0.05 cm in the mice of the control group and 0.60±0.20** cm in the treated mice (group T), respectively (Figure 1). Results revealed that the length of the testis significantly decreased (p<0.05) in the treated mice of group T as compared to the control (Figure 1).
Diameter
Regarding the diameter of the testis during the experimental period the changes were given in Figure 1. The mean diameter of the testis that was observed from the gross study were 0.40±0.02 cm in the control mice and 0.30±0.05** cm in the treated mice (group T), respectively (Figure 1). Results revealed that the diameter of the testis of group C and group D significantly decreased (p<0.05) among the treated mice in comparison to the control mice and this reduction was 25% (0.40 cm to 0.30 cm, Figure 1).
Color
Comparing to the normal testis of the control group, the treated group having a little pale colored testis.
Histo-morphological study
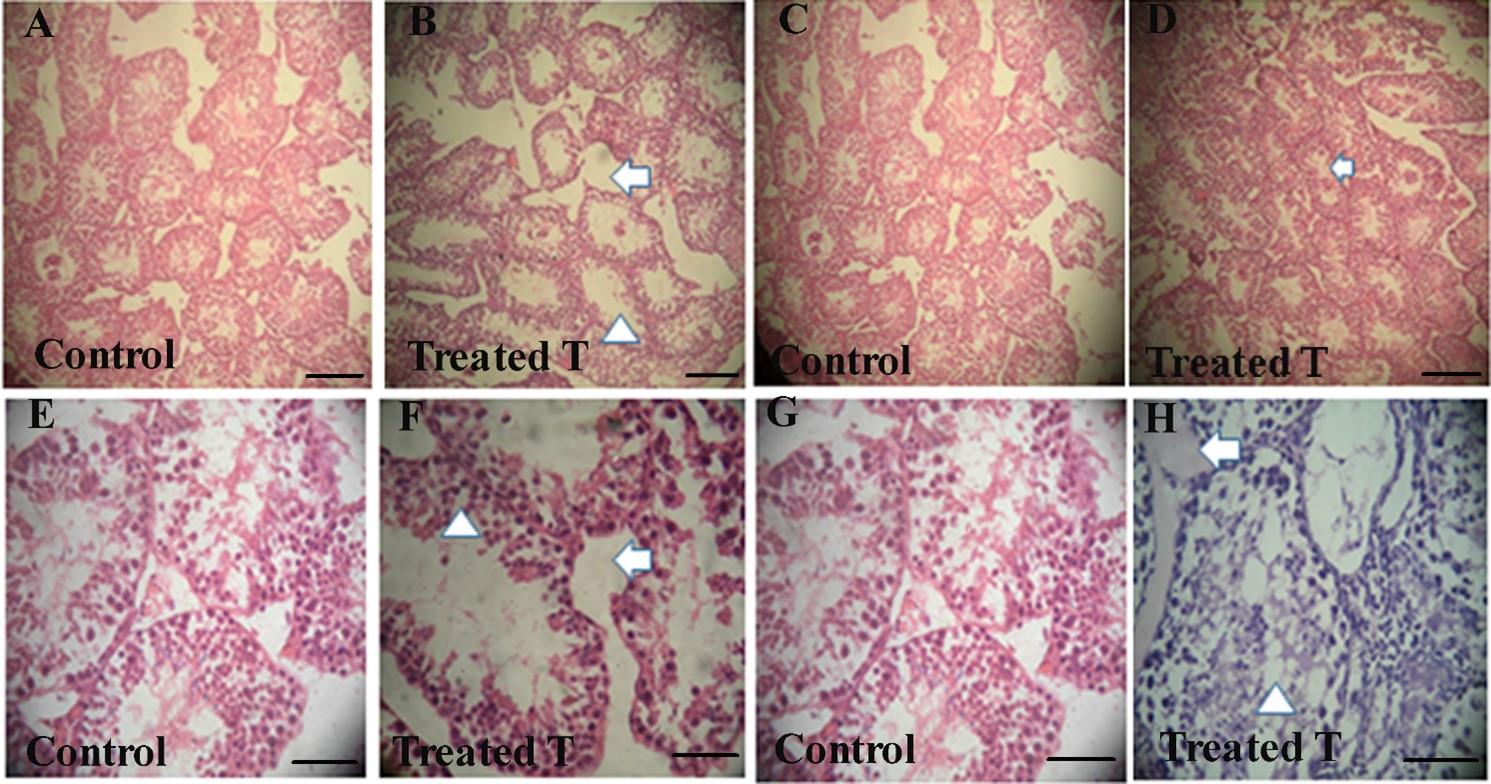
The light microscopic examination by H & E staining of testis in the tissue section of the control group C showed that the seminiferous tubules were surrounded by the connective tissue boundary as of normal histology of the testis. In a single focus, huge number of seminiferous tubules was present and the interstitial cells or the leydig cells in between the seminiferous tubules were also present. The matured spermatozoa were present within the lumen of the seminiferous tubules and the spermatocytes remained in a linear position from the periphery towards the center of the lumen. The sertoli cells were present within the seminiferous tubules (Figure 2B).
On the other hand, the histopathological changes in a single focus of the herbal extract treated testis showed that the number of the seminiferous tubules decreased in the treated group T comparing to the control (Figure 2B). In the control group, average 35 seminiferous tubules were found in a single focus whereas, in the treated groups it was 16 (Figure 2B).
The amount of the spermatozoa within the lumen of the seminiferous tubules also reduced in the treated group T comparing with the control one (Figure 2B). The amount of the spermatozoa in a single focus in the control group was 70% on an average, but in the treated group C and D it reduced to 35%. (Figure 2B).
The amount of the spermatozoa within the lumen of the seminiferous tubules decreased in the treated group T comparing with the control. In the control group (C), well organized seminiferous tubules were found but in the treated group (group T) the arrangement of the seminiferous tubules was distorted (Figure 2D).
In the treated group T, fibrous thickening of the membrane was found surrounding the seminiferous tubules and at the same time, vacuolization within the seminiferous tubules was also found in the treated group T due to the presence of the fat droplets (Figure 2F).
In the control group, huge number of the sertoli cells within the seminiferous tubules and the leydig cells in between the seminiferous tubules were found, but in the treated group T there were reduction of the number of the sertoli and Leydig cells(Figure 2H).
DISCUSSION
Results revealed that the weight of the testis significantly decreased (p<0.05) in the herbal extract treated mice of group T in comparison to the mice of the control group (C) (Figure 1). These findings were consistent with that of Ralebona et al., 2012 [19], Igweze et al., 2014 [11], Boudou et al., 2013 [26] and Mishra et al., 2008 [21]. Although, Ralebona et al.,2012 [19] and Mishra et al.,2015 [21] used the ethanolic extract of Garcinia kola and Piper nigram, respectively for their experiments. Their explanation of decreasing the weight of the testis was due to the presence of the toxic constituents of the plants.
Results revealed that the length of the testis significantly decreased (p<0.05) in the treated mice of group T as compared to the control (Figure 1). These findings resembled the observation of Igweze et al., 2014 [25] and Dehghani et al., 2012 [5]. Their explanation of decreasing the length of the testis was due to the presence of the toxic constituents of the plant. They used alcoholic extract of castor bean for their experiments and the rat as the animal model but in this present research a combination of three herbal extracts were used and the Swiss albino mice was the experimental animal.
Results revealed that the diameter of the testis of group C and group D significantly decreased (p<0.05) among the treated mice in comparison to the control mice and this reduction was 25% (0.40 cm to 0.30 cm). These findings were in consistent with the findings as described by Boudou et al., 2013 [26], but did not resemble with that of the Saganuwan et al., 2010 as they did their experiment by using the leaf of the Abrus precatorius, but in the present research we used the seed of this plant rather than the leaf.
This finding was consistent as the observation of Boudou et al., 2013 [26]. Some chemical constituents present in the plant extracts used have spermicidal effect that might be a prime cause of the reduction of the amount of the spermatozoa within the lumen of the seminiferous tubules of the treated group T. This supported the findings of Ekwere et al., 2011 [7], but they used Ricinus communis in their experiment as a single herbal plant without using it in a combination of several herbal extracts as used in the present research.
The amount of the spermatozoa within the lumen of the seminiferous tubules decreased in the treated group T comparing with the control. Similar findings were also observed as stated by of Ekwere et al., 2011 [22] and Mishra et al., 2015 [21].
In the control group (C), well organized seminiferous tubules were found but in the treated group (group T) the arrangement of the seminiferous tubules was distorted (Figure 2D). The observations of Sharaw et al., 2014 [27], Dehghani et al., 2006 [28] and Raji et al., 2006 [29] was supported by the finding of this research as well. But they did their experiment with the ethanolic extract of the Hibiscus rosasinensis and Syzygium cumini.
In the treated group T, fibrous thickening of the membrane was found surrounding the seminiferous tubules and at the same time, vacuolization within the seminiferous tubules was also found in the treated group T due to the presence of the fat droplets (Figure 2F). Similar findings were also found as described by Ekbujo et al., 2008 [30] and Galaly et al., 2014 [31] where they used the Hibiscus sandorfin (rossele) for their experiments.
In the control group, huge number of the sertoli cells within the seminiferous tubules and the leydig cells in between the seminiferous tubules were found, but in the treated group T there were reduction of the number of the sertoli and Leydig cells (Figure 2H). This histological finding supported the observation of Boudou et al., 2013 [26].
CONCLUSIONS
Considering the above mentioned results it might be praiseworthy to mention here that herbal extracts seems to hold great potential for in-depth investigation for contraception. The authors hope to attract the attention of the natural product researchers throughout the world to focus on this unexplored potential of herbal extracts, and it may be useful in developing new formulations with more therapeutic values for exploring new, affordable, available and above all safe herbal contraceptives especially in male.
ACKNOWLEDGEMENT
The research work was supported with the grants from the Ministry of Education and Ministry of Science and Technology, Bangladesh.
AUTHOR CONTRIBUTIONS
Sonali Bhakta carried out the experiments, analyzed the data and wrote the initial draft of the manuscript. Shonkor Kumar Das and Abdul Awal, designed and supervised the research work and revised the manuscript, and finalized the manuscript. The manuscript was carefully read by both the authors before the submission process.
CONFLICTS OF INTEREST
The author declares that no conflict of interest exists.
References
- [1]Gupta MK, Sharma PK, Ansari SH. In-vitro antioxidant activity of the successive extracts of Ricinus communis leaves. International Journal of Plant Sciences. 2006; 1(2): 229–231.
- [2]Michele Noonan 2013: Herbs That Affect Birth Control Pills (http://www.livestrong.com/article/375674-herbs-that-affect-birth-control-pills/).
- [3]Kadiri AB. An Examination of the Usage of Herbal Contraceptives and Abortifacients in Lagos State, Nigeria. Ethnobotanical Leaflets. 2009; 13: 140-46.
- [4]Hannah Ransom 2013. Holistic Hormonal Health and Natural Birth Control. (http://oneradionetwork.com/women-%E2%80%93-children-vaccines/hannah-ransom-holistic-hormonal-health-and-natural-birth-control-december-10-2013/).
- [5]Okoko IE, yama OE. Cutoarchitectural variations in the ovary, oviduct and uterus following intra-gastric gavages of Abrus precatorius Linn. in albino rats. Int. J. Morphol. 2011; 29(4): 408-413.
- [6]Jena J, Gupta AK. Ricinus communis Linn. A phytopharmacological review. International Journal of Pharmacy and Pharmaceutical Sciences. 2012; 4(4): 25-29.
- [7]Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Filoterapia. 2010; 81: 961-968.
- [8]Mukherjee TK. Protection of Indian traditional knowledge. Ethnomedicinal Plants. Point Publisher Jaipur 2004, pp. 1-303.
- [9]Kaur R, Sharma A, Kumar R, Kharb R. Rising trends towards herbal contraceptives. J nat. Prod. Resour. 2011; 1(4): 5-12.
- [10]Kuo SC, Chen LH. Potent antiplate let, anti-inflammatory and anti-allergic isoflavoquinones from the roots of Abrus precatorius plant. Med. 1995; 61: 307-312.
- [11]Sayeed MA, Hossain ABMM, Mondol AM, Islam MA. Antifertility studies on ethanolic extract of abrus precatorius on swiss male albino mice. International Journal of Pharmaceutical Sciences and Research. 2012; 3(1): 288-92
- [12]Raji Y, Oloyo AK, Morakinyo AO. Effect of methanol extract of Ricinus communis seed on reproduction of male rats. Asian J Androl. 2006; 8(1): 115-121.
- [13]Mark AP, Chad R, Kermit DH, David RF, Nancy KJ. Medical Aspects of Chemical and Biological Warfare (Ricin toxin). Text book of military medicine. 2007, pp. 1-607.
- [14]Bhakta S, Das SK. In praise of the medicinal plant Ricinus communis L.: a review. Global J Res. Med. Plants & Indigen. Med. 2015; 4(5): 95–105
- [15]Olsnes S, Saltvedt E, Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974; 249(3): 803-810.
- [16]Chaudhary B, Mukhopadhyay K. Syzygium cumini (L.) skeels: a potential source of nutraceuticals. International Journal of Pharmacy and Biological Sciences. 2012; 2(1): 46-53.
- [17]Muniappan A, Pandurangan S. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed. 2012; 2(3): 240–246.
- [18]Shah GM, Khan MA, Ahmad M, Zafar M, Khan AA. Observations on antifertility and abortifacient herbal drugs. African Journal of Biotechnology. 2009; 8 (9): 1959-1964.
- [19]Saganuwan SA, Onyeyili PA. Biochemical effects of aqueous leaf extract of Abrus precatorius (Jecquirity bean) in Swiss albino mice. Herba Rolonica. 2010; 56(3): 63-80.
- [20]Atuboyedia WO, Jonah SA, Chinagoro TOE. Antifertility effects of aqueous extract of Ocimum gratissimum L. leaves in male mice. Journal of Medicial plants Research. 2010; 4(9): 809-816.
- [21]Mishra RK, Singh SK. Reproductive effects of lipid soluble components of Syzygium aromaticum flower bud in male mice. Journal of Ayurveda and Integrative Medicine. 2013; 4(2): 94-98.
- [22]Ekwere EO, McNell RT, Okwuasaba FK. The effect of Ricinus communis-Linn (Ricom 1013-J) on semen parameters: a comparative study. Journal of Physics: Conference Series. 2011; 1: 7-11.
- [23]Prasenjit M, Prasanta KM, Tanaya G. Effect of Season on UV Absorbing Property of Syzygium cumini L. leaves. Glob J Pharmaceu Sci. 2018; 6(3): 555687.
- [24]Sharaw S, Ibrahim NS. The effects of Hibiscus rosasinensis flower extracts on spermatogenesis a sperm parameters of mice. Global Journal of Biology, Agriculture and Health science. 2014; 3(2): 32-35.
- [25]Igweze ZN, Orisakwe OE, Obianime AW. Reproductive parameters in a day toxicity study of smart herbal purifier-a poly herbal supplement in male rats. Journal of Applied Pharmaceutical Science. 2014; 4(10): 69-74.
- [26]Boudou F, Berroukche A, Salmi MB, Kandouci BA, Adili DEH, Tou N. Amoeliorative effects of Syzygium aromaticum essential oil on fertility in male rats exposed to manganese. Advanced in Sexual Medicine. 2013; 3: 85-91.
- [27]Dehghani F, Panjeshahin MR. The toxic effect of alcoholic extract of Citrullus colosynthis on Rat liver. Iranian Journal of Pharmacology and Therapeutics. 2006; 5(2): 117-119.
- [28]Sharma RK, Goyal AK, Yadav SK, Bhatt RA 2013. Anti-Fertility activity of Ficus religiosa fruits extract on goat uterus in vitro. Int. J. Drug Dev. & Res., 2013, 5(4): 330-335.
- [29]Ralebona N, Rusike CRS, Chungag BNN. Effects of ethanolic extract of Garcinia kola on sex behavior and sperm parameters in male Wistar rats. African Journal of Pharmacy and Pharmacology. 2012; 6(14): 1077-1082.
- [30]Ekbujo EC, Adisa OJ, Yahaya AB. A study of the staining effect of roselle (Hibiscus sabdariffa) on the histologic section of the testis. Int J. Morphol. 2008; 26(4): 927-930.
- [31]Galaly SR, Hozayen WG, Amin KA, Ramadan SM. Effects of Orlistat and herbal mixture extract on brain, testes functions and oxidative stress biomarkers in a rat model of high fat diet. Beni-Suef University Journal of Basic and Applied Sciences. 2014; 3(2): 93-105.