Comparable preventive effects of laboratory-grown spirulina and market spirulina against arsenic-induced alterations in the liver of adult rats
Abstract
Arsenic (As) is a naturally occurring ubiquitous environmental toxicant. It has been reported that spirulina has protective effects against As toxicity. In the present study, we compared the prophylactic effects of spirulina [laboratory grown agro-based spirulina (Ab-Sp) and market spirulina (M-Sp)] against the histopathological changes in liver induced by inorganic arsenic (iAs) in male rats. Three doses (1.0g, 1.5g and 2.0g/kg feed) of both the Ab-Sp and M-Sp with feed and 3.0mg NaAsO2/kg body weight (BW) in drinking water were given simultaneously to six groups (T4, T5, T6, T7, T8 and T9) of rats daily for 90 days. Same dose of NaAsO2 (3.0mg/kg; T1) and highest dose (2.0g/kg) of each of Ab-Sp (T2) and M-Sp (T3) were given individually to other 3 groups keeping the rest one as control (T0) with normal feed and water. As feeding resulted in a variety of histopathological changes in liver, including congestion in the central veins, hemorrhage in the hepatic lobules and lobular tissues, higher numbers of hypertrophic hepatocytes with hypertrophic nucleus, hepatocytes with visible chromatin in the nucleus and vacuolated hepatocytes. The Ab-Sp treatment successfully improved all the histopathological conditions. In contrast, the M-Sp improved the conditions by combating all the histopathological conditions including vacuolated hepatocytes, erosions and hemorrhages in the liver. Taken together, the spirulina was found as an effective agent in prevention of the histopathological changes while we first clarified that Ab-Sp had better result than the M-Sp and finally, 2.0g Ab-Sp/kg feed was found as the best dose.
INTRODUCTION
Arsenic (As) is a naturally occurring ubiquitous environmental toxicant widely distributed in the earth crust. Normally, As occurs in air, natural water, soil, vegetation, plants, forests and marine products and its concentrations are much higher depending on geographic locations. Arsenic occurs in both inorganic and organic forms and can exist in the environment in 4 redox states: arsenite (+3), arsenate (+5), arsine (-3) and elemental (0) forms [1]. Inorganic arsenicals have detrimental impacts on life supporting functions [2] while most of the organic arsenicals have negligible health effects and excreted unchanged [3]. Arsenite is 10 times more toxic than arsenate and 70 times more toxic than the methylated species, i.e., organic arsenicals [4].
Drinking water polluted by high level of arsenic is one of the most serious worldwide environmental problems [5]. It has been reported from at least 70 countries including 14 Asian countries that an estimated of 140 million people around the world are drinking water contaminated with arsenic exceeding 10.0 µg As/L (WHO standard; [6,7]. Although, drinking of high arsenic contaminated water is the primary pathway of arsenic exposure to humans [8,9], arsenic can also enter into the human food chain directly or indirectly due to its wide spread distribution in both the plant and animal kingdoms [10]. As a result, it has been reported that 85.0 million peoples are at risk of arsenic toxicity for consuming both arsenic rich drinking water and foodstuffs in Bangladesh [11,12].
Arsenicosis caused serious public health problems including melanosis, leukomelanosis, hyperkeratosis, black foot disease, hepatomegaly, neuropathy, cancer and gangrene [9], weakness, anemia, burning sensation of eyes, liver fibrosis, chronic lung disease, bone marrow depression [13], vascular remodeling, portal hypertension, noncirrhotic liver fibrosis [14].
Arsenic causes histopathological changes in tissues and organs of the exposed subject. The hepatotoxic action of arsenic includes cirrhotic portal fibrosis [15], hepatomegaly, non-cirrhotic portal fibrosis and portal hypertension [16,14], oxidative damage of liver [14,17], fatty accumulation, parenchymal cell degeneration [18], steatosis, hepatocyte degeneration [19], hepatic fibrosis [14], and liver proliferative lesions [18]. Fibrosis of liver may progress to cirrhosis and even to liver cancers [20]. Necrosis of hepatocytes and cytoplasmic blebbing and expanded sinusoidal spaces due to shrinkage and necrosis of hepatocytes were found on arsenite-exposure [21]. However, there were significant vascular remodeling with increased sinusoidal endothelial cell capillarization, vascularization of the peribiliary vascular plexus and constriction of hepatic arterioles [22]. Few focal areas of necrosis, Kupffer cell hyperplasia, and localized fibrosis in the periportal region were observed [23]. Ballooning of hepatocytes in the periportal and parenchymal areas, chromatin fragmentation and drop-out necrosis [24], and swollen hepatocytes near the centrilobular vein were observed in liver [25].
However, the first important step to control arsenicosis is the cessation of drinking arsenic contaminated water [17] and ingesting arsenic rich foods. Then, supplementation of potential antioxidants and feeding of arsenic burden reducing agent seem to be beneficial for remedy of arsnicosis [26,27]. Antioxidant supplement may have preventive effects in arsenicosis [28,29,26]. Intake of cysteine, methionine, niacin, vitamin B12 and choline facilitates arsenic methylation by modulating its metabolism [30].
Spirulina (Spirulina platensis), a blue-green alga, has been considered as an excellent whole food ever known to mankind [31,32] having antioxidant properties [33,34]. It has the corrective properties against heavy metal toxicity, nephrotoxicity induced by heavy metals and drugs and also against cancer, tumor growth and malnutrition [32,35]. As arsenic induces the generation of ROS [36], alters DNA methylation pattern in many genes [37] and also inactivate enzymes in the cellular energy pathway [38], as a result, antioxidants could be helpful in altering the cytotoxicity of arsenic [39]. β-carotene is a very important antioxidant [40] and β-carotene from spirulina pose more antioxidant properties than synthetic one. Phycocyanin, a pigment of spirulina, stimulates the immune system and assists detoxification with the ability to inhibit oxidative damage in DNA [41]. Spirulina is rich in protein with all the essential amino acids, antioxidants, galaxy of phytonutrients and polysaccharides that trigger enzyme systems to enhance detoxification of arsenic [42]. Spirulina contains a lot of minerals including zinc, which is essential for a strong immune system and for powering antioxidant enzyme systems and acts synergistically with β-carotene. Micronutrients of Spirulina interact with toxic metals at several points in the body like absorption and excretion of toxic metals; transport of metals in the body; binding to target proteins; metabolism and sequestration of toxic metals; and in secondary mechanisms of toxicity such as oxidative stress [43]. Hence, spirulina intake can reduce the effects of arsenicosis by modulating its methylation, reducing oxidative stress, binding to target proteins, enhancing arsenic excretion from the body, and finally by reducing susceptibility to arsenicosis by combating malnutrition.
Therefore, this work has been taken with a view to evaluate the histopathological changes induced by inorganic arsenic and to clarify the comparative effects of laboratory grown agro-based spirulina (Ab-Sp) and market spirulina (M-Sp) in prevention of histopathological alterations in liver.
MATERIALS AND METHODS
Animals
Apparently healthy 40 male Long Evans rats with minimum of 350.0g BW were selected and randomly divided into 10 groups consisting of 4 rats in each group. The rats of all groups, except that of T0, T2 and T3 groups, were fed with NaAsO2 (Merck, Darmstadt, Germany: 0.58 mg iAs/mg) at 3.0 mg/kg BW/day in drinking water. Simultaneously, the rats of T4, T5 and T6 group were individually given 3 doses of the Ab-Sp at 1.0g, 1.5g and 2.0g/kg feed respectively while that of the T7, T8 and T9 group were similarly supplied M-Sp at 1.0g, 1.5g and 2.0g/kg feed respectively in feed. The rats of T2 were treated with only 2.0g Ab-Sp/kg feed while the T3 group rats were treated with the same dose of M-Sp (2.0g/kg feed) in feed as Ab-Sp and M-Sp treated control respectively. The rats of the T1 group were fed only with 3.0 mg NaAsO2/kg BW/day in drinking water as arsenic treated control whereas; the rats of T0 group were supplied only normal feed and drinking water as non-treated control. The trial was continued for 90 days. All experimental protocols were approved by the Animal Welfare and Ethical Committee, Faculty of Veterinary Science, Bangladesh Agricultural University. All efforts were made to minimize the number of mice used and their sufferings.
Feeding trial
A 0.2% NaAsO2 stock solution was prepared with deionized water and preserved at 4°C to feed the trial rats for use of maximum 7 days. Dried powder of Ab-Sp obtained from the laboratory production and the dried powder of M-Sp collected from the local market. Required amounts of the respective doses (1.0g, 1.5g and 2.0g/kg feed) of both the Ab-Sp and M-Sp were individually mixed with pellet feed and dried at 50°C in an electric oven for at least 20 hours. Then, the spirulina mixed feed was taken into air tight polypropylene container for supplying to the trial rats for 5 days from the preparation. A new lot of spirulina mixed feed was prepared generally 12 hours before the 5th day from the previous feed preparation.
In every morning, required amount of the prepared NaAsO2 solution (0.2%) for the rats of a group per day at the rate of 3 mg As/kg BW/day was calculated and taken into a previously washed and sterilized waterer. Then, a small amount of drinking water was added to the solution so as not to exceed the half of the daily requirement of drinking water for the rats of that particular group. This was done to ensure drinking of the total amount of NaAsO2 solution within 6 to 8 hours of supply. After drinking the total amount of NaAsO2 solution, the rats were allowed to drink normal drinking water ad libitum. This As feeding process was done for the rats of T1, T4, T5, T6, T7, T8 and T9 groups. Concomitantly to the feeding of NaAsO2 solution, the spirulina mixed feed with the respective doses of the individual spirulina (Ab-Sp and M-Sp) were supplied ad libitum to the rats of all six groups and the rats of T2 and T3 groups. This feeding process was continued for 90 days.
Sample collection
On Day 90, all the trial rats were euthanized with high dose of chloroform (Fisher Scientific UK Limited, UK) and samples were collected by opening the carcass immediate after euthanization. An intact piece of liver was collected from the sample rats. The collected samples were immediately washed in buffered neutral formalin and taken into individual stoppered glass vial filled with adequate amount of buffered neutral formalin (10 x sample volume) for fixation and kept at room temperature until processed for histopathological investigation.
After 48 hours, the fixed tissues were trimmed with a sharp blade into a suitable size and shape (about 0.5cmX0.5cm). Immediately after trimming, the liver was preserved in fresh buffered neutral formalin into new clean and dried stoppered glass vials.
Staining of tissue sections and photomicrograph
Routine hematoxylin and eosin (H & E) staining was used for the tissue sections. Preparation of stains and other necessary chemical solutions, and staining procedures were described previously [44]. Tissue sections were examined under compound microscope and photographs of tissue sections were taken under a Differential Interference Contrast (DIC) Microscope.
Examination of liver sections
The degree of changes, if any in the hepatocytes, was expressed by the number of hepatocytes with changes in respect to the total number following counting of hepatocytes.
Statistical analysis
The designs of the experiments were Randomized Complete Block Design (RCBD). The data were tabulated into a preliminary data sheet of a computer and compared with computer spread sheets to ensure the accuracy of the data. Then the data were analyzed with computer programs: Microsoft Excel and SPSS (Statistical Package for Social Science), and also using the analysis of variance technique by a computer using of MSTAT computer package program in according to the principles of RCBD. Least Significance Difference (LSD) was done to compare the variations between treatments where ANOVA showed significant differences.
RESULTS
Overall histopathological findings
With routine H&E staining, the liver showed normal in structures with mitotic phases in few nuclei, although a considerable number of hepatocytes showed hypertrophic hepatocytes with hypertrophic nucleus and hepatocytes with visible chromatin in the rats of all the trial groups in a variable degree (Table 1). In the rats of the T1 group, the liver was found with vacuolated hepatocytes, blood accumulation in some of the central veins, and erosions of lobular epithelium, and consequently hemorrhages in some hepatic lobules and lobular tissues as extravasations while no vacuolated hepatocytes, erosion and hemorrhage was found in rats of other trial groups (Figure 1A-D).
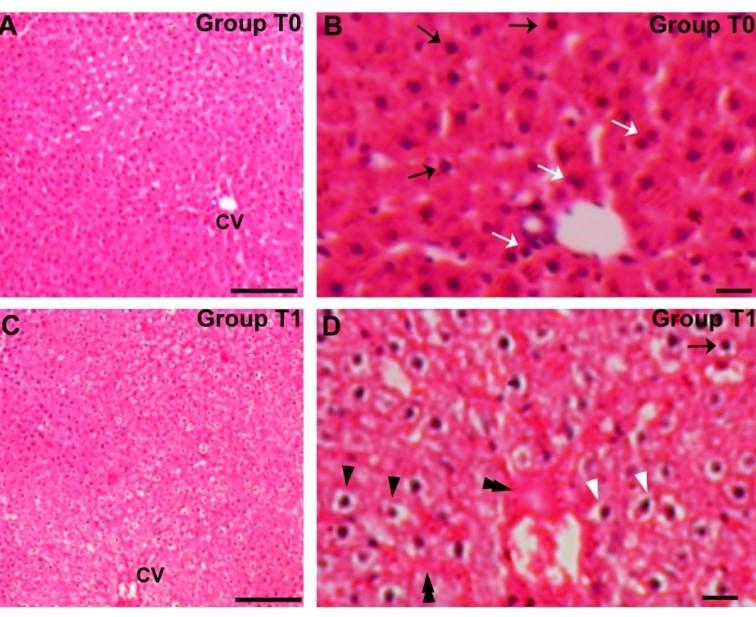
Table 1. Incidence of hypertrophic hepatocytes with hypertrophic nucleus and hepatocytes with visible chromatin in the nucleus of the liver of trial rats.
Hypertrophic hepatocytes with hypertrophic nucleus in the liver
The livers of the T8 group rats had the highest numbers of hypertrophic hepatocytes with hypertrophic nucleus while the lowest number was in the T0 group. However, the number of hypertrophic hepatocytes with hypertrophic nucleus in the liver of the rats of all of the groups differed significantly (p<0.01) among the groups. Arsenic induction significantly increased the numbers of hypertrophic hepatocytes with hypertrophic nucleus in the liver compared to the control. However, the numbers of that type of cell between T0 and T5, and among T1, T2, T3, T6 and T7 groups did not vary significantly (Table 1). The findings (based on percent values) show that none of the doses of any of the spirulina improved this histopathological condition at the control level. However, the lowest and intermediate doses of the Ab-Sp decreased (T4: 19.60% and T5: 48.57%) the number of the hypertrophic hepatocytes with hypertrophic nucleus in the liver of the As induced rats compared to the T1 group, but none of the M-Sp doses could do (Table 1).
Hepatocytes with visible chromatin in the nucleus in the liver
The rats of the T7 group showed the highest numbers of the hepatocytes with visible chromatin in the nucleus while the lowest number of that was in the T5. However, the numbers of the hepatocytes with visible chromatin in the nucleus varied significantly (p<0.01) among the trial groups. Although, the numbers of that type of hepatocytes did not differ significantly between the T1 and T2, among the T0, T4, T5 and T6 groups, and among the T3, T7, T8 and T9 groups. The data (based on percent values) show that all of the M-Sp doses increased compared to both T0 and T1 while all of the Ab-Sp doses decreased this histopathological condition at the control level. However, the average decreasing rate of the Ab-Sp was 34.50% vs. 67.41% compared to T0 vs. to T1 and the dose of 1.5g Ab-Sp/kg feed (52.57% vs. 76.40% decreased compared to T0 vs. to T1) was found best in reducing the numbers of hepatocytes with visible chromatin in the liver of the As induced rats (Table 1).
DISCUSSION
In the present study we the first to compare the effect of Ab-Sp and M-Sp on the histopathological changes in liver induced by iAs in rats. iAs induced variety of histopathological changes in liver tissues and spirulina was found effective in prevention of these histopathological changes.
In the present study the livers of the trial rats with arsenic dosing alone resulted in vacuolated hepatocytes, erosions of the lobular epithelium, and hemorrhages in some of the central veins and hepatic lobules including lobular tissues as extravasations. In accordance to the present findings, similar hemorrhages were found to be frequent throughout the liver [21] hepatocyte with hypertrophy and fatty infiltration as widespread vacuoles consistent with fatty droplets were observed after chronic arsenic exposure [18, 21]. However, the spirulina feeding with arsenic in rats resulted in full recovery from these conditions and the Spirulina treatments without arsenic in rats did not induce such histological changes in the liver. Besides these, hypertrophic hepatocytes with hypertrophic nucleus and hepatocytes with visible chromatin were found in the liver of rats of all the groups with variable degrees.
Arsenic feeding alone (T1) and treatments of both the spirulina without arsenic (T2 and T3) showed significantly higher (p<0.01) numbers of both hypertrophic hepatocytes with hypertrophic nucleus and hepatocytes with visible chromatin in the livers of the trial rats compared to the control. This finding indicates that arsenic dosing and both the spirulina treatments without arsenic in the rats induced the increased numbers of both hypertrophic hepatocytes with hypertrophic nucleus and hepatocytes with visible chromatin in the livers of rats above the control level.
The numbers of both hypertrophic hepatocytes with hypertrophic nucleus and hepatocytes with visible chromatin in the livers of the trial rats in all the As plus Ab-Sp groups were found lower compared to all of the As plus M-Sp groups as well as T1 groups. But, the numbers of the hypertrophic hepatocytes with hypertrophic nucleus were higher while the numbers of the hepatocytes with visible chromatin were lower in all the As plus Ab-Sp groups compared to the control. However, none of the M-Sp doses was found to reduce the numbers of both the types of the hepatocytes compared to the control and T1 groups. These findings reveal that all the doses of the Ab-Sp improved both these histological conditions from the intensity caused by arsenic dosing in the liver. It was evident that all the Ab-Sp doses decrease the numbers of the hepatocytes with visible chromatin below the control level, although the numbers of the hypertrophic hepatocytes with hypertrophic nucleus were decreased with all the Ab-Sp doses but could not return at the control level with any of the Ab-Sp doses. The Ab-Sp treatment with arsenic at the dose of 1.5g Ab-Sp/kg feed (T5) was found as the best dose among Ab-Sp doses in reducing the numbers of both types of the hepatocytes. On the other hand, none of the doses of the M-Sp reduce the number of any type of the cells at the control level and even at the T1 group level.
In conclusions, In the present study, we the first to clarify the comparative effects of Ab-Sp and M-Sp. Arsenic induced histopathological changes in liver were fully or partially recovered by spirulina feeding to the arsenic induced rats, and the Ab-Sp was found better in majority of the cases than the M-Sp in prevention of the changes.
ACKNOWLEDGEMENTS
The authors are thankful to the United States Department of Agriculture, USA, for financial support (Grant No. USDA/31/2006, BGARS-117) through the research project entitled “Detection of arsenic in the food chains and animal samples and study the preventive measure using the best cost-effective agricultural products-based spirulina against arsenicosis in man and livestock” under the Department of Pharmacology, Bangladesh Agricultural University, Mymensingh, Bangladesh. The authors also grateful to the Field Fertility Clinic (FFC) Laboratory, Department of Surgery and Obstetrics, BAU, Mymensingh for supporting the study providing DIC microscope.
AUTHOR CONTRIBUTION
AK, MAA and MZIK designed the experiment, AK, MRI, MRJ performed experiments, AK analyzed the data. AK drafted the manuscript. MZIK and MNI critically revised the manuscript.
CONFLICT OF INTERSEST
The authors declare no conflict of interest.
References
- [1]Oremland RS, Stolz JF 2003: The ecology of arsenic. Available at: http//www.sciencemag.org/cgi
- [2]Mahimairaja S, Bolan NS, Adriano DC, Robinson B. Arsenic contamination and its risk management in complex environmental settings. Adv Agron. 2005; 86: 1-82.
- [3]Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. Journal of American Medical Association. 2008; 300: 814-822.
- [4]Squibb KS Fowler BA: The toxicity of arsenic and its compounds. In: BA Fowler (Editor), Biological and Environmental Effects of Arsenic, Elsevier, Amsterdam. 1983; pp. 233-269.
- [5]IARC 2004: International Agency for Research on Cancer, Some drinking-water disinfectants and contaminants, including arsenic, IARC, Lyon, France. 84 pp. 41-267.
- [6]Mukherjee AB, Bhattacharya P, Jacks G, Banerjee DM, Ramanathan AL, Mahanta C, Chandrashekharam D, Chatterjee D and Naidu R. Groundwater arsenic contami nation in India: Extent and severity. In: R Naidu, E Smith, G Owens, P Bhattacharya, P Nadebaum (Editors), Managing Arsenic in the Environment: From Soil to Human Health. CSIRO Publishing, Melbourne, Australia. 2006; pp. 553–593.
- [7]Bagchi S: Arsenic threat reaching global dimensions. Can Med Assoc J. 2007; 177(11): 1344–1345.
- [8]Smith AH, Arroyo AP, Mazumder DN, Kosnett MJ, Hernandez AL, Beeris M, Smith MM, Moore LE. Arsenic-induced skin lesions among Atacameno people in Northern Chile despite good nutrition and centuries of exposure. Environ Health Perspect. 2000; 108: 617–620.
- [9]Kapaj S, Peterson H, Liber K, Bhattacharya P. Human health effects from chronic arsenic poisoning- A Review. J Environ Sci Heal A. 2006; 41: 2399-2428.
- [10]Kile ML, Houseman AE, Breton CV, Smith T, Quamruzzaman Q, Rahman M, Mahiuddin G, Christiani DC. Dietary arsenic exposure in Bangladesh. Environ Health Perspect. 2007; 115(6): 889-93.
- [11]Hossain, MF Arsenic contamination in Bangladesh—an overview. Agr Ecosyst Environ. 2006; 113(1-4): 1-16.
- [12]Wahidur R .Arsenic Exposure in Bangladesh: The Reproductive and Developmental Health Effects in Humans. Philadelphia Annual Meeting held on 22–25 October, 2006; Paper No. 67-3.
- [13]Ng JC, Wang J, Shraim A. A global health problem caused by arsenic from natural sources. Chemosphere 2003; 52: 1353-1359.
- [14]Mazumder DN. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol Appl Pharm. 2005; 206(2): 169-75.
- [15]Santra A, Das Gupta J, De BK, Roy B, Guha Mazumder DN. Hepatic manifestations in chronic arsenic toxicity. Ind l of Gastrol. 1999; 18: 152-155.
- [16]Santra A, Maiti A, Das S, Lahiri S, Charkaboty SK, Mazumder DN. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J. Toxicol.Clin. Toxicol. 2000; 38: 395-405.
- [17]Das, NK, Sengupta SR. Arsenicosis: Diagnosis and treatment. Indian Journal of Dermatology, Venereology and Leprology 2008; 74: 571-581.
- [18]Chen H, Li S, Liu J, Diwan BA, Barrett JC, Waalkes MP. Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation: implications for arsenic hepatocarcinogenesis. Carcinogenesis 2004; 25: 1779–1786.
- [19]Wu J, Liu J, Waalkes MP, Cheng ML, Li L, Li CX, Yang Q: High dietary fat exacerbates arsenic-induced liver fibrosis in mice. Exp Biol Med. 2008; 233: 377-384.
- [20]Centeno JA, Mullick FG, Martinez L, Page NP, Gibb H, Longfellow D, Thompson C, Ladich ER. Pathology related to chronic arsenic exposure. Environ Health Perspect. 2002; 110 (Suppl. 5): 883–886.
- [21]Ferzand R, Gadahi JA, Saleha S, Ali Q Histological and haematological disturbance caused by arsenic toxicity in mice model. Pak J Biol Sci. 2008; 11(11): 1405-1413.
- [22]Straub AC, Stolz DB, Ross MA, Hernández-Zavala A, Soucy NV, Klei LR, Barchowsky A. Arsenic Stimulates Sinusoidal Endothelial Cell Capillarization and Vessel Remodeling in Mouse Liver. Hepatology. 2007; 45(1): 205–212.
- [23]Mandal AK, Das S, Basu MK, Chakrabarti RN, Das N: Hepatoprotective Activity of Liposomal Flavonoid against Arsenite-Induced Liver Fibrosis. J Pharmacol Exp Ther. 2007; 320: 994–1001.
- [24]Bashir S, Sharma Y, Irshad M, Gupta SD, Dogra TD. Arsenic-induced cell death in liver and brain of experimental rats. Bas Clin Pharmacol Toxicol. 2006; 98: 38–43.
- [25]Carmignani M, Boscolo P, Iannaccone A. Effects of chronic exposure to arsenate on the cardiovascular function of rats. Br J Ind Med. 1983; 40: 280–284.
- [26]Khandker S, Dey RK, Islam AZM, Ahmad SA, Ifthaker-Al-Mahmud. Arsenic-safe drinking water and antioxidants for the management of arsenicosis patients. Bangl J Pharmacol. 2006; 1: 42-50
- [27]McCall MR, Balz F. Can antioxidant vitamins materially reduce oxidative damage in humans. Free Radical Bio Med. 1999; 26: 1034–1053.
- [28]Lee TC, Ho IC. Differential cytotoxic effects of arsenic on human and animal cells. Environ Health Perspect. 1994; 102: 101–105.
- [29]Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003; 79(933): 391-396.
- [30]Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, Parvez F, Baron JA Howe GR, Ahsan H. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am. J. Clin. Nutr.. 2007; 85(5): 1367-1374.
- [31]Switzer L. Spirulina, the whole food revolution. Proteus Corporation, USA. 1980; pp. 1-69.
- [32]Johson PE, Shubert LE. Accumulation of mercury and other elements by Spirulina (cyanophyceae ). Nutr. Rep. Int. 1986; 34: 1063-1071.
- [33]Benedetti SF, Benvenuti S, Pagliarani S, Francogli S, Scoglio, Canestrari F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004; 75: 2353-2362.
- [34]Dartsch PC. Antioxidant potential of selected Spirulina platensis preparations. Phytother Res. 2008; 22(5): 627-633.
- [35]Belay A, Ota Y, Miyakawa K, Shimamatsu H. Production of high quality Spirulina at Earthrise Farms. In: Phang et al (Editors), Proceedings of the Algal Biotechnology in the Asia-Pacific Region, 1994, University of Malaya, Malaysia. 1994; pp 92-102.
- [36]Barchowsky A, Klei LP, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low level of arsenite. Free Radical Bio Med. 1999; 27: 1405-1412
- [37]Chanda S, Dasgupta UB, Guha DN, Guha Mazumder DN, Gupta M, Chaudhuri U, Lahiri S, Das S, Ghosh N, Chatterjee D. DNA hypermethylation of promoter of gene p53 and p16 in arsenic-exposed people with and without malignancy. Toxicol. Sci. 2006; 89(2): 431-437.
- [38]Vahidnia A, van der Voet GB, Wolff de FA. Arsenic neurotoxicity — a review. Hum. and Ex. Toxicol. 2007; 26: 823-832.
- [39]Hei TK, Filipic M. Role of oxidative damage in the genotoxicity of arsenic.Free Radical Bio. Med. 2004; 37(5): 574-581.
- [40]Schwartz J, Flynn E, Shklar G. The effect of carotenoids on the antitumor immune response in vivo and in vitro with hamster and mouse immune effectors. In: A Bendich, R Chandra, K Gerard, A Cerami, F Takaku (Editors) Micronutrients and immune functions – Cytokines and metabolism. New York Academy of Sciences. 1990; pp. 92-109.
- [41]Bhat VB, Madyastha KM. Scavening of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: protection against oxidative damage to DNA. Biochem. Biophys. Res. Commun. 2001; 285: 262-266.
- [42]Dasgupta T, Banerjee S, Yadav PK, Rao AR. Chemomodulation of carcinogen metabolizing enzymes, antioxidant profiles and skin and fore stomach papillomagenesis by Spirulina platensis. Mol. Cell. Biochem. 2001; 226: 27-38.
- [43]. Marjorie MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT. Effects of micronutrients on metal toxicity. Environ. Health Perspect. 1998; 10: 203-216.
- [44]Jannat N, Amin T, Sultana N, Jahan MR, Islam MR: Long term administration affects hemato-biochemical parameters and liver architecture of swiss Albino mice. J Adv Biotechnol Exp Ther. 2018; 1(2):29-35.