Pharmacological effect of methanolic and hydro-alcoholic extract of Coconut endocarp
Abstract
Cocos nucifera used as natural remedies in a wide variety of diseases. The current experiment aimed to determine the qualitative phytochemicals, anxiolytic, anti-diarrheal, anti-inflammatory, thrombolytic and cytotoxic actions of C. nucifera endocarp, which extracted by using methanol (MeOH-CNE) and the hydro-alcohol (HaE-CNE). The MeOH-CNE and HaE-CNE subjected to phytochemical screening, where both extracts showed the existence of secondary metabolites such as carbohydrates, flavonoids, cardiac glycosides, and proteins. The anxiolytic activity screened by elevated plus maze experiment, whereas the percentage of open arm accounts 74.55 ± 4.54%, 66.31 ± 4.41% at 400 mg/kg for HaE-CNE, MeOH-CNE respectively (P < 0.05) while the standard drug Diazepam (75.24 ± 3.91%). In the anti-diarrheal test, extracts (200 and 400 mg/kg) and standard drug loperamide (5 mg/kg) showed dose-dependent significant (P < 0.05) inhibition against castor oil-induced diarrhea. The MeOH-CNE and HaE-CNE exhibited 72.91 ± 4.20 %, 64.42 ± 5.50% inhibition of protein denaturation at 500 μg/ml, while in the thrombolytic test, HaE-CNE showed the highest and significant (P < 0.05) in clot lysis activity on human blood in comparison to water, whereas streptokinase used as standard. In brine shrimp lethality bioassay, the LC50 of HaE-CNE and MeOH-CNE ware 432.35 µg/ml and 1173.88 µg/ml respectively. The LC50 for standard vincristine sulfate was 43.15 µg/ml. The current results suggested that MeOH-CNE and HaE-CNE have promising pharmacological activity.
INTRODUCTION
Cocos nucifera (L.) belongs to the family Arecaceae (palm family), which is frequently termed a tree of life. For many years, coconut products have used as folk medicine. In ayurvedic medicine, coconut products such as oil, milk, cream, and water are being castoff in the treatment of hair loss, burns and heart complications [1]. All parts of C. nucifera can be used traditionally in various pathological conditions such as shell fibers of Cocos nucifera are used in diarrhea [2], antipyretic, renal inflammation and as a cream for dermatitis, sores, and injuries. The fibers are also used in asthma and diabetes. Moreover, the leaves and roots parts are used in diarrhea and stomach pains. The solid albumen of coca extracted as oil, pulp, milk which are used in antipyretic, diarrheal treatment, preventing hair loss, wound healing [3, 4]; oral contraceptive, diarrheal treatment, aphrodisiac, get rid of from skin rash affected by HIV infection, respectively. The white surface of the Coca is used to consider for fever and malaria. The water of C. nucifera is used in treatment of renal disease [4-7]. Several pharmacological and biological activities are identified in several reports, including analgesia; anti-inflammatory, antimicrobial, antioxidant, anti-osteoporosis, anti-diabetic, anti-neoplastic, anthelminthic, antihypertensive, vasodilation, defense of kidney, heart, and liver functions and anti-malarial activities [8-12].
Anxiety syndromes are the most usual mental, emotional, and behavioral complications [13] affecting 264 million peoples around the globe [14]. In terms of overall management and treatment, depression among mood disorders has always been problematic [15]. Stress, depression and anxiety might trigger insomnia. Medicinal plants have been taken as sleep aids in insomnia throughout the world [16]. Despite the development of more molecules against the treatment of depression, unfortunately this disease remains untreated in many patients because of the burden of their side effects [17]. Therefore, searching for new therapeutic agents having no adverse effects may be the option of improved pharmacological actions, higher efficacy and safety.
Diarrheal syndromes are a key problem in underdeveloped countries and are accountable for the loss of a huge number of people every year [18]. Diarrhea is characterized by an increase in the water content, volume, or incidence of stools [19]. The research shown that several microbes like Salmonella, Escherichia coli, Vibrio cholerae and Shigella generate and release enterotoxins which are the major cause of diarrhea in developing countries [20]. Opioids and its derivatives such as difenoxin and loperamide are mostly used in the management of diarrhea [21]. Medicinal plants are an effective source for developing new antidiarrheal drugs. Therefore, the WHO has encouraged using medicinal plants in the management of diarrhea [22]. Currently available opioids and its derivatives are linked with several adverse effects for example abdominal pain, distention, bloating, nausea, vomiting, and constipation [23] and also cardiotoxicity has been testified by loperamide [24].
Endocarp of C. nucifera is the hardest part of the fruit and a rich source of phenolic and flavonoid content [25]. According to the literature review, C. nucifera shell has phenolic content [26]. The study aimed to investigate the in vivo anxiolytic, antidiarrheal and in vitro anti-inflammatory and thrombolytic, cytotoxic effects along with the phytochemical study of methanolic and hydro-alcoholic extract of C. nucifera endocarp. The present study investigates the in vivo anxiolytic, antidiarrheal and in vitro anti-inflammatory, thrombolytic, cytotoxic effects of C. nucifera, a Bangladeshi plant for the first time.
MATERIALS AND METHODS
Chemicals
Loperamide hydrochloride, castor oil (Sigma-Aldrich, MO, USA), Streptokinase (Beacon Pharmaceutical Ltd., Mymensingh, Bangladesh), Sea salt non ionized NaCl, vincristine sulphate (Sigma-Aldrich, MO, USA), ethanol, Tween 80 were used in this study. All other chemicals and reagent were used of analytical grade.
Preparation of plant extract
The moist endocarp of C. nucifera were collected from the Sitakunda local area of Chittagong, Bangladesh, which later validated by Professor Dr. Sheikh Bokhtear Uddin, Department of Botany, University of Chittagong. The C. nucifera endocarp layer was collected in a fresh condition. Then these are cut into small pieces if necessary to make it suitable for grinding purposes. Then the crude parts were rubbed for ten days and later grounded to powder by a mechanical drier (Ecocell, MMM Group, Germany) at 55-60 °C. By using another mechanical grinder (NOWAKE, Japan), the samples were ground to a coarse powder. The powder (500 g.) was soaked in 500 ml of methanol and 70% ethanol, respectively, for a week with occasional shaking and stirring on a shaker machine at room temperature. It was then filtered through a cotton plug followed by Whatman filter paper No. 1. After filtration, the filtrate was evaporated by using a rotary evaporator at 50 °C under reduced pressure to obtain the methanol crude extract (MeOH-CNE) (9.5 g) and hydroalcoholic extract (HaE-CNE) (11.48 g). The extracts were conserved at 4 °C in a refrigerator until further use.
Experimental animals
Swiss Albino mice (Six-seven weeks old) of either sex were purchased from the International Center for Diarrheal Diseases Research, Dhaka, Bangladesh (ICDDRB). The animals were adapted to the laboratory condition (25 ± 2 °C with a light/dark cycle of 12 h) for seven days before the study. The study was conducted following approval by the Institutional Animal Ethical Committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh, under the reference number (P&D-147/13-18).
Phytochemical screening
The qualitative phytochemical screening was executed according to the regular procedures [27] and the results shown presence or absence of secondary plant metabolites such as alkaloids, flavonoids, tannins, saponins, phenol, carbohydrate and glycoside.
Anxiolytic activity
Elevated plus maze test
Anxiolytic activity assessed by an elevated plus maze (EPM) apparatus, which used for the unlearned response. The EPM apparatus elevated from the floor at 40 cm, where it consists of two open arms and closed arms with a central square [28, 29]. The negative control received 1% Tween-80 (10 ml/kg, b.w), the reference drug Diazepam received (1 mg/kg, b.w) intraperitoneally, while the treatment group received 200 mg/kg and 400 mg/kg, b.w. by oral gavage. After sixty minutes, each mouse positioned in the center of the EPM facing towards the closed arm. Entry into open and closed arm recorded for 5 minutes and calculated the percentage by the resulting equation: % of open arm entries=(Number of entries in open arm)/(Number of entries in open arm+number of entries in closed arm ) ×100
Anti-diarrheal activity
Castor oil-induced diarrhea
The castor-oil induced diarrhea was followed to evaluate the antidiarrheal according to the method described by Taufiq et al. and Bellah et al. [30, 31]. Mice of either sex fasted for 24 hours. The negative control received 1% Tween-80 (10 ml/kg, b.w), the reference drug loperamide (5 mg/kg, b.w; as oral suspension), while the treatment group received 200 mg/kg and 400 mg/kg, b.w. by oral gavage. After 60 minutes, each mouse received 0.5 ml castor oil orally by gavage and individually placed in cages consist of transparent paper. The transparent paper changed in every hour and counted dry and wet feces in every 60 min for 4 hours. The equation calculated the level of % inhibition of defecation: % inhibition of defecation= (A-B)/A ×100
Where, A = average eradication feces number of the control group; B = average eradication feces number of the text group.
Anti-inflammatory activity
Inhibition of protein denaturation method
The anti-inflammatory activity of the extracts determined using the protein denaturation method [32, 33]. The constituents of the reaction solution were 100 μl of the extracts (final concentration 62.5-500 μg/ml) and 100 μl of 5% aqueous bovine serum albumin. Then the pH was adjusted using glacial acetic acid. The samples were incubated at 37 °C for 20 min and then heated to 70 °C for 10 min. After incubation, the mixture was allowed to cool for 10 min, and a turbid solution found. Then the turbidity was measured at 660 nm. The blank consist of the sample and distilled water. Distilled water was used as negative control. The positive control was diclofenac sodium. Percentage inhibition was calculated using the formula:
% of protein denaturation = [(absorbance of control – absorbance of test sample) / (absorbance of test control)] × 100
Thrombolytic activity
The clot lysis activity performed as described by Prasad et al. [34]. A 3 ml venous blood is withdrawn from the ten healthy volunteers who did not have any previous history of taking NSAID or contraceptives. The withdrawn blood was distributed in nine different pre-weighed sterile micro centrifuge tubes (0.5 ml/tube) and incubated for 45 min at 37 °C. After the formation of the clot, completely removed the serum without disturbing the clot and each tube reweighed for calculating the clot weight. The plant extracts ware added to each micro centrifuge tube separately containing pre-weighed clot. 100 µL of streptokinase and distilled water were added separately to the positive and negative control group. All the tubes were then incubated at 37 °C for 90 min and observed for clot lysis. The released fluid was removed and reweighed the tube to calculate the difference in weight after clot disruption.
% of clot lysis = (weight of clot after remove of fluid/clot weight) × 100
Brine shrimp cytotoxicity
The brine shrimp lethality bioassay experiment was followed by the previous reported method [35]. In the artificial seawater (3.8% NaCl solution), the shrimp eggs hatched for 48 hours for maturing the shrimp called nauplii. The shrimp egg collected from the Katabon, Dhaka. The crude extract was dissolved in DMSO to obtain a solution of 5 mg/ml, which was subjected to serially diluted concentrations of 20 to 100 μg/ml, followed by the addition of 5.0 ml of artificial seawater in each test tubes. Ten of the living nauplii applied to each of all experimental vials and control vials. Following 24 hours, all vials inspected by an amplifying glass, and the number of living nauplii in each vial was observed and recorded. Experiments were conducted in a set of triplicate manner along with reference drug vincristine sulfate. The lethal concentration (LC50) that would kill one-half of the nauplii was determined from a linear regression equation.
% of mortality = (N0-N1/N0) ×100
Where, N0= the number of nauplii taken; N1= the number of nauplii alive.
Statistical analysis
Result represented as Mean ± Standard Error Mean (SEM). P (< 0.05 and < 0.001) were measured as statistically significant while the Dunnet’s test was used to describe the significance using GraphPad Prism Version 6.0 (GraphPad software Inc., San Diego, CA).
RESULTS
Phytochemical analysis revealed the presence of secondary metabolites
The qualitative phytochemical screenings of MeOH-CNE and HaE-CNE have performed to find out the presence or absence of secondary plant metabolites. In our results, both extracts showed the presence of carbohydrates, flavonoids, cardiac glycosides, and protein. Whereas, only HaE-CNE showed the presence of alkaloids (Table 1).
Table 1. Qualitative phytochemical screening of MeOH-CNE and HaE-CNE.
Both the extracts reveled significant anxiolytic activity in EPM test
In elevated plus-maze (EPM) test, both extract reveled significant (P < 0.05) anxiolytic activity compared to the control group. The HaE-CNE treated mice at dose of 400 mg/kg showed significant (P < 0.05) increase of the percentage of open-arm entries (74.55 ± 4.54) compared to the control group, whereas the MeOH-CNE treated mice showed 66.31 ± 4.41 percentage of open-arm entries at the same dose (Figure 1). On the other hand, the reference drug diazepam at a very small dose 1 mg/kg, b.w treated mice produced a noticeable increase in the percentages of open-arm entries (75.24 ± 3.91).
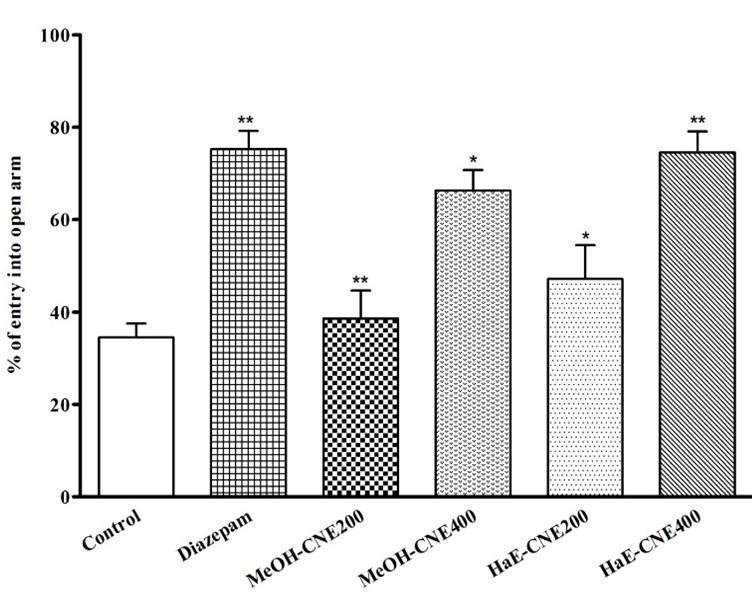
Both the extracts reduces the rate of defecation in a time-dependent manner
In the castor oil-induced diarrheal experiment, both extracts were found to be effective and significant (P < 0.05) in a dose dependent manner on experimental mice at all tested doses. At the dose of 400 mg/kg the HaE-CNE showed 56.79 ± 2.33% reductions in the rate of defecation in albino mice. Whereas, the MeOH-CNE extract showed 51.23 ± 3.26% reductions at the same dose. This condition was markedly reduced (62.49 ± 1.11%) by standard drug Loperamide at a dose of 5 mg/kg Figure 2.
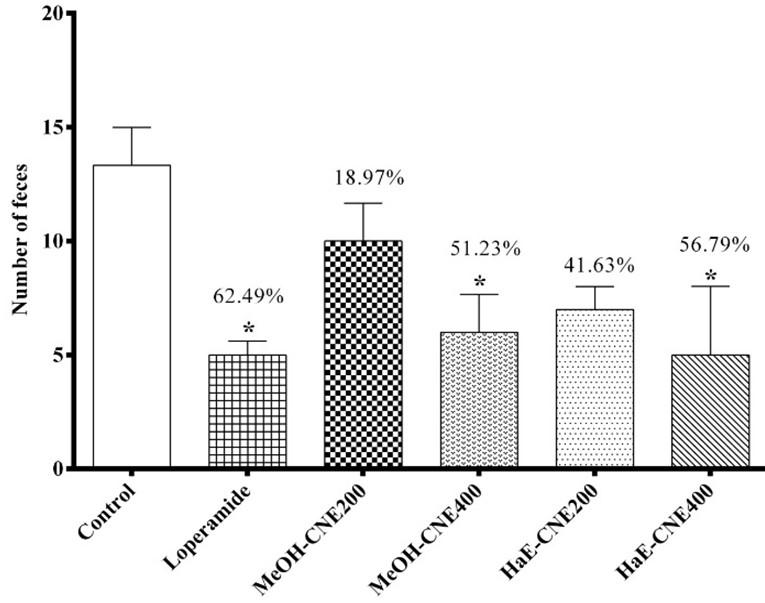
Both the extracts increases the inhibition of protein denaturation
The results of anti-inflammatory activity of the extract are displayed in Figure 3. The result showed a dose dependent and significantly (P < 0.05) increased the inhibition of protein denaturation by MeOH-CNE, HaE-CNE and Diclofenac-Na throughout the concentration range (62.5-500 μg/ml). The crude hydro alcoholic extract (HaE-CNE) demonstrated maximum inhibition of protein denaturation (72.91 ± 4.20%) at 500 μg/ml, whereas the methanolic extract (MeOH-CNE) showed 64.42 ± 5.50% inhibitions at the same concentration.
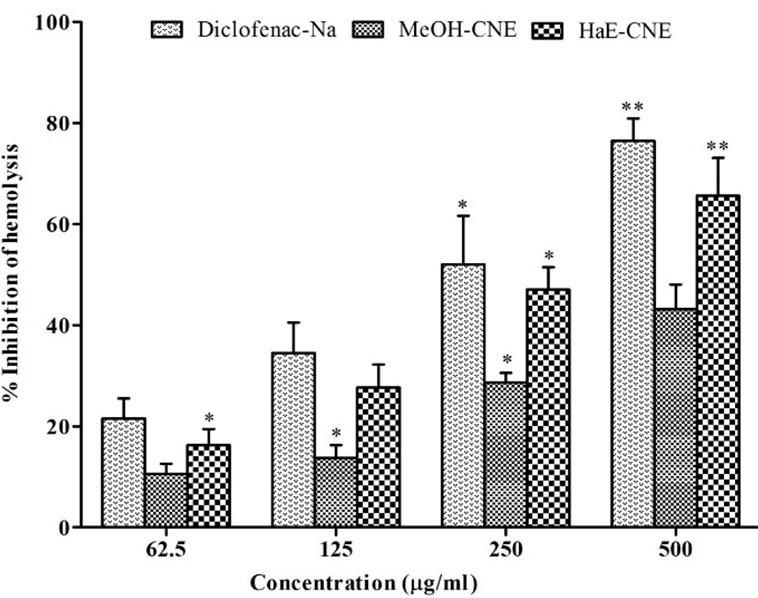
Both the extracts significantly increases clot lysis activity
The results of clot lysis activity of extracts are shown in Figure 4. After addition of 100 μl of Streptokinase a positive control (15,00,000 I.U.) to the clots along with 90 minutes of incubation at 37 °C, showed 73.44 ± 1.87% clot lysis. When clots were treated with negative control (sterile distilled water), a negligible clot lysis (8.87 ± 1.32) has observed. After the treatment of clots with HaE-CNE and MeOH-CNE, the significant (P < 0.05) clot lysis was observed. The clot lysis activity of MeOH-CNE and HaE-CNE ware 35.73 ± 3.21% and 55.74 ± 2.78% respectively.
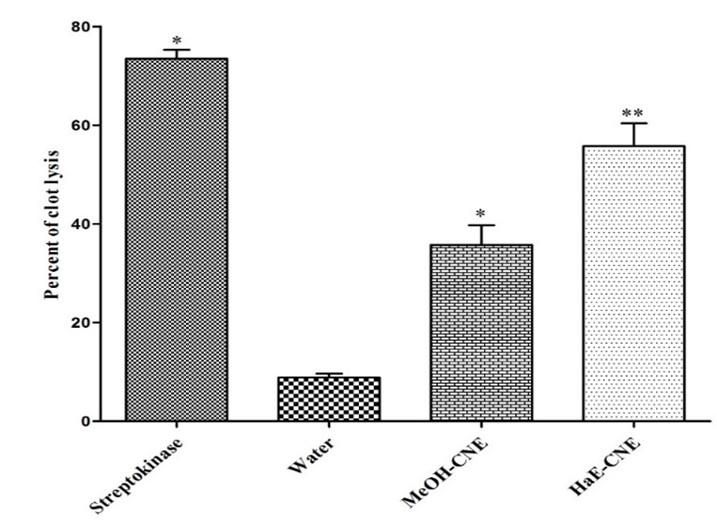
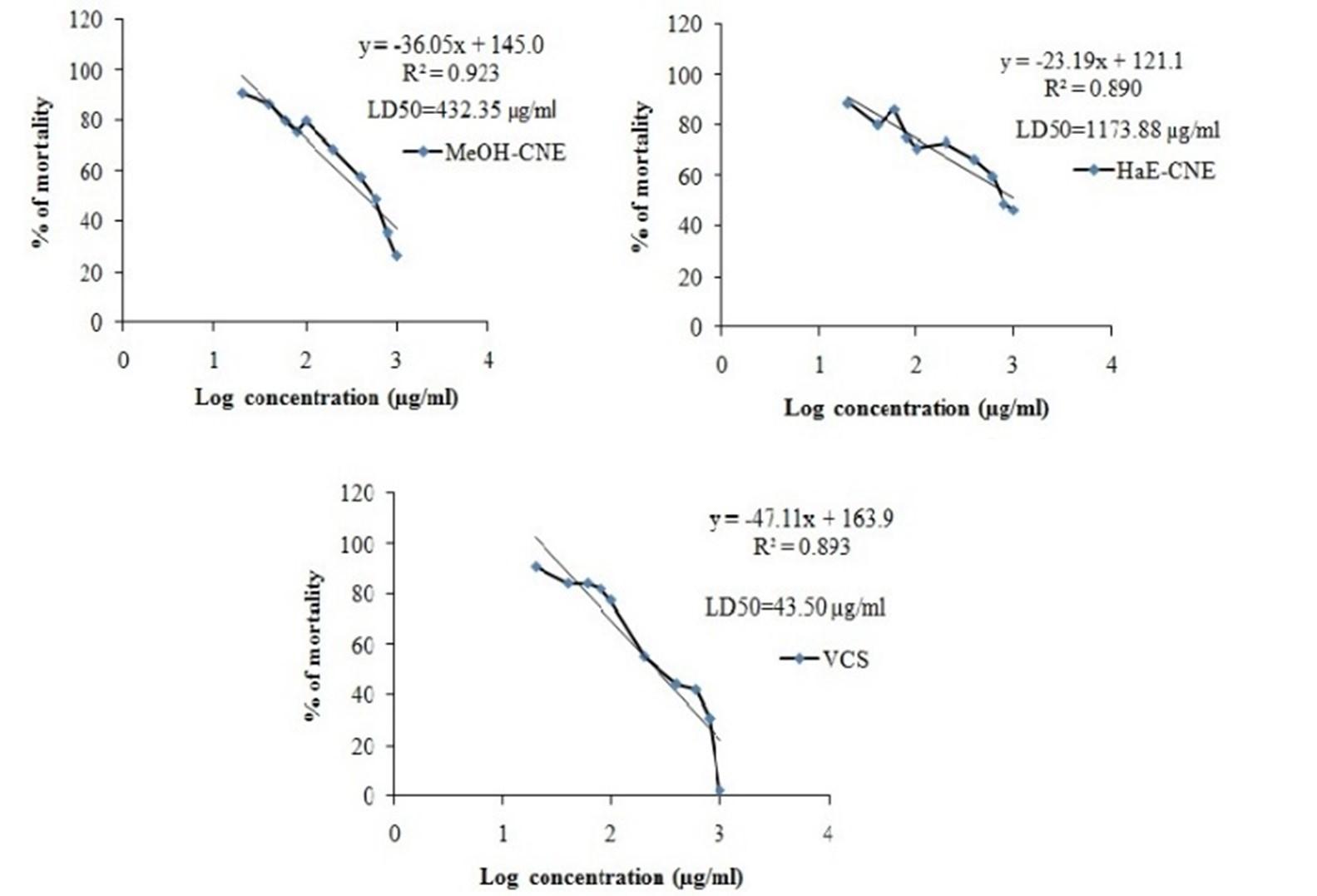
Both the extracts significantly decreases the mortality of brine shrimp
In brine shrimp lethality bioassay, the rate of mortality of nauplii was presented in Figure 5. The degree of lethality shown by the extracts was found to be directly proportional to the concentration of the extract ranging from the lowest concentration (20 µg/ml) to the highest concentration (1000 µg/ml). Both extract virtually non-toxic on the brine shrimp. They showed very low toxicity, giving LC50 values greater than 100 μg/ml. The LD50 values of MeOH-CNE, HaE-CNE and vincristine sulfate (VCS) were 432.35 µg/ml 1173.88 µg/ml and 43.15 µg/ml, respectively.
DISCUSSION
The phytochemical analysis led on the plant extracts shown the presence of compound which is referred to show therapeutic as well as biological activities. Analysis of plant extract shows the presence of alkaloids, carbohydrates, flavonoids, cardiac glycosides, and protein. The phytochemical investigation led on the plant extracts shown the presence of compound which is referred to show therapeutic as well as physiological activities [36]. The current findings are similar to the previous study of the ethanolic extract of mesocarp [4].
The elevated plus maze (EPM) test is considered to be a good test procedure to evaluate the anxiety-like behavior [28, 37]. The increasing number of entries in the open arm indicates the anxiolytic effects and the increasing number of entries at the closed arm indicates the anxiogenic effects [37, 38]. Administration of MeOH-CNE, HaE-CNE showed an increased amount of entries in the open arms, an indication of anxiolytic-like behavior. Similar observations found to the reference drug (diazepam) which significantly increased the percentage of open arm entries. The current findings are similar to the previous study of ethanolic extract of C. nucifera endocarp, whereas the 500 mg/kg dose exhibited 74.77 ± 6.86 percentages of open arm entries [39].
The assessment of C. nucifera endocarp on castor oil-induced diarrheal mice to exhibit the dose-dependent manner, especially HaE-CNE showed a significant (P < 0.05) amount of reduction of diarrhea in comparison to the negative control. Similar observations found to the reference drug (Loperamide) which significantly increased the reduction of diarrheal faces. Castor oil is a recognized diarrheal agent due to the presence of ricinoleic acid which causes changes in the intestinal mucosa, as a result, fluid and watery luminal content that flow rapidly by intestine [40]. Due to the release of ricinoleic acid, it produces different inflammatory mediators such as prostaglandins, nitric oxide, platelet-activating factor, cAMP, and histamine [41, 42]. The present result validating the traditional use and the presence of secondary metabolites flavonoids [43].
The assay of anti-inflammatory effects was the possible effect of MeOH-CNE, HaE-CNE on protein denaturation assay. Inflammation causes lysis of lysosomes which release their enzyme and produces a variety of diseases. NSAIDs exhibit their activity by inhibiting the release of lysosomal enzymes [44]. Denaturation of proteins is initiated by inflammatory processes [45]. Thus, NSAIDs provide protection against protein denaturation. So, the ability of MeOH-CNE, HaE-CNE to inhibit the protein denaturation may provide a significant contribution to its anti-inflammatory properties. The present result might be responsible for the presence of secondary metabolites such as alkaloid and flavonoid [46].
The available thrombolytic drugs in the market, particularly streptokinase, convert plasminogen to plasmin and increased clot lysis. According to the literature review explained that flavonoids, among the plant metabolites, affect thrombosis and cardiovascular disease by interfering with platelet activation. Cocos nucifera endocarp, especially HaE-CNE, showed a significant (P < 0.05) amount of clot lysis in comparison to the negative control. This result might be because of the presence of flavonoids which influence embolus and cardiovascular disorder by interfering with platelet actuation [47].
Brine shrimp lethality assay is used for the cytotoxicity study. Ideally, the potential agent for the treatment of cancer should be nontoxic to a normal cell. However, anticancer agents are every so often lethal to normal cells, particularly towards rapidly growing cells. It is important to test this extract in low concentration to assess its potency [48]. The results observed in 24 h were found to be nontoxic for extracts. Generally, the smaller the LC50, the higher the toxicity, and vice versa. The value of LC50 over 1000 µg/ml is considered to be nontoxic, ranging from 500 -1000 µg/ml is weakly toxic, moderately toxic for 100 – 500 µg/ml while less than 100 µg/ml is considered as highly toxic [49-69]. No extracts were found to be toxic compared to Vincristine sulfate. The presence of alkaloids and saponins are present in this study, which can be effective as cytotoxic agents [27]
CONCLUSIONS
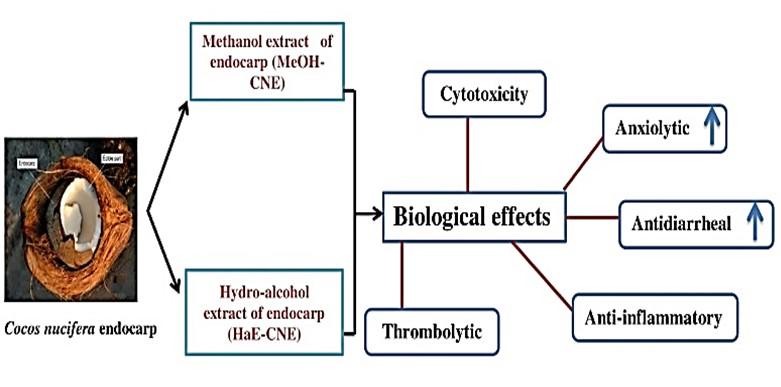
Coconut endocarp extract can be regarded as a promising candidate with high therapeutic potential for drug preparation (Figure 6). The current study may provide useful data concerning the different medicinal properties in coconut shells for protecting humans against common diseases. From the above results, it may be concluded that the endocarp extract of C. nucifera exhibited secondary metabolites with a dose-dependent manner anti-inflammatory activity and moderate thrombolytic activity with lower cytotoxicity. Moreover, significant anxiolytic and antidiarrheal effects observed by both methanolic and hydro-alcoholic extract of C. nucifera endocarp. Therefore, further work, especially bioassay-guided fractionation, is warranted in order to isolate and characterize the active constituents responsible for the specific biological property.
ACKNOWLEDGEMENT
Authors wish to thank the authority of International Islamic University Chittagong for their kind support in progress of the research. We also grateful to Laboratory of Alternative Medicine and Natural Products, Biochemistry and Molecular Biology, University of Chittagong for providing the facility to conducted this research. The authors are also thankful to Dr. Sheikh Bokhtear Uddin, Professor, Department of Botany, University of Chittagong, for identifying the plant.
AUTHOR CONTRIBUTIONS
SA, MS, AMT, MSN, MAR, ASMAR, ZMB and MAH together planned and designed the research. ASMAR, MAR and TBE arranged the whole facilities for the research and supervised the whole research. SA, MS, AMT, MSN and MJR conducted the entire laboratory works with ZMB and MAH. MAR, ASMAR and TBE imparted in study design and interpreted the results putting efforts on statistical analysis and also participated in the manuscript draft and has thoroughly checked and revised the manuscript for necessary changes in format, grammar and English standard. All authors read and agreed on the final version of the manuscript.
CONFLICTS OF INTEREST
Authors declared that they have no conflict of interest.
References
- [1]DebMandal M, Mandal S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention2011.
- [2]Esquenazi D, Wigg MD, Miranda MM, Rodrigues HM, Tostes JB, Rozental S, da Silva AJ, Alviano CS. Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res Microbiol. 2002;153:647-52.
- [3]Sachs M, Asskali F. Wound management with coconut oil in Indonesian folk medicine. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 2002;73:387-92.
- [4]Lima EBC, Sousa CNS, Meneses LN, Ximenes NC, Santos Júnior MA, Vasconcelos GS, Lima NBC, Patrocínio MCA, Macedo D, Vasconcelos SMM. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Brazilian Journal of Medical and Biological Research. 2015;48:953-64.
- [5]Nagata JM, Jew AR, Kimeu JM, Salmen CR, Bukusi EA, Cohen CR. Medical pluralism on Mfangano Island: use of medicinal plants among persons living with HIV/AIDS in Suba District, Kenya. J Ethnopharmacol. 2011;135:501-9.
- [6]Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Amran AA, Mahmud R. Evaluation of the use of Cocos nucifera as antimalarial remedy in Malaysian folk medicine. J Ethnopharmacol. 2011;134:988-91.
- [7]Emojevwe V. Cocos nucifera (Coconut) fruit: A review of its medical properties. 2013;3:718-23.
- [8]] Akinpelu DA, Alayande KA, Aiyegoro OA, Akinpelu OF, Okoh AI. Probable mechanisms of biocidal action of Cocos nucifera Husk extract and fractions on bacteria isolates. BMC Complement Altern Med. 2015;15:116.
- [9]Rinaldi S, Silva DO, Bello F, Alviano CS, Alviano DS, Matheus ME, Fernandes PD. Characterization of the antinociceptive and anti-inflammatory activities from Cocos nucifera L. (Palmae). J Ethnopharmacol. 2009;122:541-6.
- [10]Silva RR, Oliveira e Silva D, Fontes HR, Alviano CS, Fernandes PD, Alviano DS. Anti-inflammatory, antioxidant, and antimicrobial activities of Cocos nucifera var. typica. BMC Complement Altern Med. 2013;13:107.
- [11]Jose M, Sharma BB, Shantaram M, Ahmed SA. Ethnomedicinal herbs used in oral health and hygiene in coastal Dakshina Kannada. J Oral Health Comm Dent. 2011;5:119-23.
- [12]Jose M, Cyriac MB, Pai V, Varghese I, Shantaram M. Antimicrobial properties of Cocos nucifera (coconut) husk: An extrapolation to oral health. J Nat Sci Biol Med. 2014;5:359-64.
- [13]Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health. 2008;29:115-29.
- [14]Hannah Ritchie, Roser M. Mental Health. Our World in Data. 2019.
- [15]Rex A, Schickert R, Fink H. Antidepressant-like effect of nicotinamide adenine dinucleotide in the forced swim test in rats. Pharmacology Biochemistry and Behavior. 2004;77:303-7.
- [16]Guzmán-Gutiérrez SL, Balderas J, Aguilar A, Navarrete A. Sedative activity of some plants used in Mexico to treat insomnia. Rev Latinoamer Quím. 2009;37:243-51.
- [17]Pawar VS, Anup A, Shrikrishna B, Shivakumar H. Antidepressant–like effects of Acorus calamus in forced swimming and tail suspension test in mice. Asian Pacific Journal of Tropical Biomedicine. 2011;1:S17-S9.
- [18]Shoba FG, Thomas M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmacol. 2001;76:73-6.
- [19]Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, Griffin PM, DuPont H, Sack RB, Tarr P, Neill M, Nachamkin I, Reller LB, Osterholm MT, Bennish ML, Pickering LK. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331-51.
- [20]Tenório JA, Dulciana S, da Silva TM, da Silva TG, Ramos CS. Solanum paniculatum root extract reduces diarrhea in rats. Revista Brasileira de Farmacognosia. 2016;26:375-8.
- [21]Hilal-Dandan R, Brunton L. Goodman and Gilman manual of pharmacology and therapeutics: McGraw Hill Professional; 2013.
- [22]Mekonnen B, Asrie AB, Wubneh ZB. Antidiarrheal activity of methanolic leaf extract of Justicia schimperiana. Evidence-Based Complementary and Alternative Medicine. 2018;2018:10.
- [23]Hanauer SB. The role of loperamide in gastrointestinal disorders. Rev Gastroenterol Disord. 2008;8:15-20.
- [24]Salama A, Levin Y, Jha P, Alweis R. Ventricular fibrillation due to overdose of loperamide, the “poor man’s methadone”. Journal of community hospital internal medicine perspectives. 2017;7:222-6.
- [25]Bankar GR, Nayak PG, Bansal P, Paul P, Pai KSR, Singla RK, Bhat VG. Vasorelaxant and antihypertensive effect of Cocos nucifera Linn. endocarp on isolated rat thoracic aorta and DOCA salt-induced hypertensive rats. Journal of ethnopharmacology. 2011;134:50-4.
- [26]Rodrigues S, Pinto GA. Ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder. Journal of Food Engineering. 2007;80:869-72.
- [27]Tiwari P, Kumar B, Kaur M, Kaur G, Kaur HJIps. Phytochemical screening and extraction: a review. 2011;1:98-106.
- [28]Almeida RNdJEGK, Rio de Janeiro, Brasil. Psicofarmacologia: fundamentos práticos. 2006.
- [29]Costa de Melo N, Sánchez-Ortiz BL, Dos Santos Sampaio TI, Matias Pereira AC, Pinheiro da Silva Neto FL, Ribeiro da Silva H, Alves Soares Cruz R, Keita H, Soares Pereira AM, Tavares Carvalho JC. Anxiolytic and antidepressant effects of the hydroethanolic extract from the leaves of Aloysia polystachya (Griseb.) Moldenke: A study on zebrafish (Danio rerio). Pharmaceuticals (Basel, Switzerland). 2019;12:106.
- [30]Taufiq-Ur-Rahman M, Shilpi JA, Ahmed M, Hossain CF. Preliminary pharmacological studies on Piper chaba stem bark. Journal of Ethnopharmacology. 2005;99:203-9.
- [31]Bellah SF, Islam MN, Karim MR, Rahaman MM, Nasrin MS, Rahman MA, Reza AA. Evaluation of cytotoxic, analgesic, antidiarrheal and phytochemical properties of Hygrophila spinosa (T. Anders) whole plant. Journal of Basic and Clinical Physiology and Pharmacology. 2017;28:185-90.
- [32]Sakat S, Juvekar AR, Gambhire MN. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci. 2010;2:146-55.
- [33]Ansari P, Uddin MJ, Rahman MM, Abdullah-Al-Mamun M, Islam MR, Ali MH, Reza AA. Anti-inflammatory, anti-diarrheal, thrombolytic and cytotoxic activities of an ornamental medicinal plant: Persicaria orientalis. Journal of Basic and Clinical Physiology and Pharmacology. 2017;28:51-8.
- [34]Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thrombosis Journal. 2006;4:14.
- [35]Rahman MA, Sultana R, Emran TB, Islam MS, Rahman MA, Chakma JS, Rashid H-u, Hasan CMMJBc, medicine a. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement Altern Med. 2013;13:25.
- [36]Mandal S, Patra A, Samanta A, Roy S, Mandal A, Mahapatra TD, Pradhan S, Das K, Nandi DK. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pacific journal of Tropical Biomedicine. 2013;3:960-6.
- [37]Ahmed S, Rakib A, Islam MA, Khanam BH, Faiz FB, Paul A, Chy MNU, Bhuiya NMA, Uddin MMN, Ullah SA. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clin Phytosci. 2019;5:36.
- [38]Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164-70.
- [39]Patil V, V.M C, Hugar S. Evaluation of anti-anxiety activity of Cocos nucifera endocarp on anxiety models. Asian Journal of Pharmacy and Pharmacology. 2019;5.
- [40]Rizzo V, Clifford MN, Brown JE, Siracusa L, Muratore G. Effects of processing on the polyphenol and phenolic acid content and antioxidant capacity of semi‐dried cherry tomatoes (Lycopersicon esculentum M.). Journal of the Science of Food and Agriculture. 2016;96:2040-6.
- [41]Rahman MA, bin Imran T, Islam S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi Journal of Biological Sciences. 2013;20:213-25.
- [42]Khalid S, AdilShahzad N, Muhammad A, Anwar P. Phytochemical screening and analysis of selected medicinal plants in Gujrat. Journal of Phytochemistry and Biochemistry. 2018;2:108.
- [43]Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: A Review 2011.
- [44]Mounnissamy VM, Kavimani S, Balu V, Quine SD. Evaluation of anti-inflammatory and membrane stabilizing property of ethanol extract of Cansjera rheedii J. Gmelin (Opiliaceae). Iranian Journal of Pharmacology and Therapeutics. 2007;6:235-0.
- [45]Umapathy E, Ndebia E, Meeme A, Adam B, Menziwa P, Nkeh-Chungag B, Iputo J. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. Journal of Medicinal Plants Research. 2010;4:789-95.
- [46]Shravan K, Kishore G, Siva K, Sindhu P. In vitro anti-inflammatory and anti-arthritic activity of leaves of Physalis angulata L. Int J Pharm Ind Res. 2011;1:211-3.
- [47]Dwivedi S. Terminalia arjuna Wight & Arn.—a useful drug for cardiovascular disorders. Journal of Ethnopharmacology. 2007;114:114-29.
- [48]Chowdhury TA, Kamal AM, Chowdhury KAA, Jahan A, Hossain MS, Mamur A, Hasan M, Hossain J. Cytotoxic & thrombolytic activity of methanolic extract of Macaranga denticulata Bark. The Pharma Innovation. 2015;4:36.
- [49]Nguta J, Mbaria J, Gakuya D, Gathumbi P, Kabasa J, Kiama S. Biological screening of Kenyan medicinal plants using Artemia salina (Artemiidae). Pharmacologyonline. 2011;2:458-78.
- [50]Rakib A, Ahmed S, Islam MA, Haye A, Uddin SMN, Uddin MMN, Hossain MK, Paul A, Emran, TB. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci Nutrition. 2020;8(1):547-556.
- [51]Shifah F, Tareq AM, Sayeed MA, Islam MN, Emran TB, Ullah MA, Mukit MA, Ullah M. Antidiarrheal, cytotoxic and thrombolytic activities of methanolic extract of Hedychium coccineum leaves. J Adv Biotechnol Exp Therapeutics. 2020;3(1):77-83.
- [52]Dutta T, Paul A, Majumder M, Sultan RA and Emran TB. Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem Biophy Rep. 2019;21:1-8.
- [53]Bulbul MRH, Rahman MA, Rahman MZ, Emran TB, Afroze M, Khan M, Chowdhury MAH, Ibrahim MA and Chowdhury MS (2019). Alcoholic extract of Leea macrophylla (Roxb.) root reverses CCl4 induced liver injury through upregulation of antioxidative enzyme’s gene mRNA expression: a molecular interaction for therapeutic inception. Oriental Pharmacy and Experimental Medicine. pp. 1-18.
- [54]Uddin MZ, Rana MS, Hossain S, Dutta E, Ferdous S, Dutta M and Emran TB (2019). In vivo neuroprotective, antinociceptive, anti-inflammatory potential in Swiss albino mice and in vitro antioxidant and clot lysis activities of fractionated Holigarna longifolia Roxb. bark extract. Journal of Complementary and Integrative Medicine. 17(2):1-9.
- [55]Emran TB, Dash R, Uddin MMN, Rahman MA. Sedative, anxiolytic, antinociceptive, anti-inflammatory and antipyretic effects of a chloroform extract from the leaves of Urena sinuata (Borss) L. in rodents. J Appl Life Sci Int. 2018;16(3): 1-19.
- [56]Uddin MMN, Ahmed S, Kabir MSH, Rahman MS, Sultan RA, Emran TB. (2017). In vivo analgesic, anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic fruits extract from Daemonorops robusta Warb. Journal of Applied Pharmaceutical Sciences. 7 (1): 104-113.
- [57]Al Mahmud Z, Qais N, Bachar SC, Hasan CM, Emran TB and Uddin MMN (2017). Phytochemical investigations and antioxidant potential of leaf of Leea macrophylla (Roxb.). BMC Res Notes. 2017;10: 245.
- [58]Al Mahmud Z, Emran TB, Qais N, Bachar SC, Sarker M and Uddin MMN (2016). Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb). Journal of Basic and Clinical Physiology and Pharmacology. 27(1):63-70. DOI: 10.1515/jbcpp-2015-0056.
- [59]Dash R, Ahsan MT, Hosen SMZ, Rahman MG, Emran TB and Uddin MMN. Evolution of selective COX-2 inhibitor from Alangium salvifolium: an in silico approach. J Appl Pharm Sci. 2015;5(4):89-93.
- [60]Kabir MSH, Hossain MM, Kabir MI, Rahman MM, Hasanat A, Emran TB and Rahman MA. Phytochemical screening, antioxidant, thrombolytic, α-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia K. Koch leaves. J Young Pharmacists. 2016;8(4):391-397.
- [61]Uddin MMN, Zahan S, Islam MA, Ahmed S, Tajbiha-E-Mowla, Rahman MS, Sultan RA and Emran TB. Evaluation of the anti-diarrheal activity of methanol extract and its fractions of Urena sinuata L. (Borss) leaves. J Appl Pharm Sci. 2016;6(12):056-060.
- [62]Dash R, Emran TB, Paul A, Siddique MK, Khan MA, Rahman MG and Uddin MMN. (2016). Effects of five Bangladeshi plant extracts on in vitro thrombolysis and cytotoxicity. Pharmacognosy Research. 8(3):176-180.
- [63]Emran TB, Rahman MA, Uddin MMN, Rahman MM, Uddin MZ, Dash R and Layzu C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis. BMC Complement Altern Med. 2015;15:128.
- [64]Emran TB, Rahman MA, Uddin MMN, Dash R, Hossen MF, Mohiuddin M and Alam MR. Molecular docking and inhibition studies on the interactions of Bacopa monnieri’s potent phytochemicals against Staphylococcus aureus. DARU J Pharm Sci. 2015;23:26.
- [65]Rahman MA, Mahmud S, Akhter S, Aklima J, Akhter S, Merry SR, Jubair SMR, Dash R and Emran TB (2015). Anti-thrombotic effects of five organic extracts of Bangladeshi plants and in silico models for the mechanism of the observed effects. Evidence-Based Complementary and Alternative Medicine.
- [66]Dash R, Ahsan MT, Hosen SMZ, Rahman MG, Emran TB and Uddin MMN (2015). Evolution of selective COX-2 inhibitor from Alangium salvifolium: an in silico approach. Journal of Applied Pharmaceutical Sciences. 5(4): 89-93.
- [67]Momin MAM, Bellah SMF, Rahman SM, Rahman AA, Murshid GMM and Emran TB. Phytopharmacological evaluation of ethanol extract of Sida cordifolia L. roots. Asian Pac J Trop Biomed. 2014;4(1):18-24.
- [68]Biswas FB, Roy TG, Rahman MA, Emran TB. An in vitro antibacterial and antifungal effects of cadmium(II) complexes of hexamethyltetraazacyclotetradecadiene and isomers of its saturated analogue. Asian Pac J Trop Biomed. 2014;7(Suppl. 2):S534-S539.
- [69]Emran TB, Rahman MA (2014). Sedative, anxiolytic and analgesic effects of Urena sinuata L. leaf extract in animal models. International Food Research Journal. 21(5):2069-2075.