Antidiarrheal, cytotoxic and thrombolytic activities of methanolic extract of Hedychium coccineum leaves
Abstract
The study reports the in vivo antidiarrheal and in vitro cytotoxic and thrombolytic activities of methanolic extract of Hedychium coccineum leaves (MEHCL). The antidiarrheal activity was evaluated by castor oil-induced diarrhea, whereas the intestinal motility by charcoal marker. In addition, brine shrimp lethality bioassay and human blood clot lysis were used to evaluate the cytotoxic and thrombolytic activities, respectively. In antidiarrheal study, castor oil-induced diarrhea and gastrointestinal motility exhibited a significant dose dependent reduction in diarrhea and defecation and an extremely significant (P < 0.0001) inhibition in intestinal motility and peristalsis index by 200 and 400 mg/kg of MEHCL. The brine shrimp lethality bioassay revealed a considerable cytotoxic effect of MEHCL (LC50= 81.59 µg/mL; R² = 0.927) while in thrombolytic a significant percentage of clot lysis (17.36%, P < 0.01) demonstrated. The findings suggest that H. coccineum leaves could be potential sources for biological activity.
INTRODUCTION
Diarrhea is one of the major infectious diseases in third world countries for the child [1], which caused by disturbances in secretion and absorption of the intestine, causing increased volume rate of feces [2]. As indicated by the World Health Organization (WHO), Bangladesh is one of the susceptible to children-diarrhea, while 17% of Bangladeshi children (<5 years) admitted in the pediatric ward [1, 3]. Acute and chronic type diarrhea may occur where acute diarrhea caused due to epidemiological reasons such as traveling. The chronic type of diarrhea lasting more than four weeks [4, 5]. Around 88% of deaths identified with diarrheal are because of insufficient sanitation and poor cleanliness while the primary causative agent for diarrhea is S. flexneri, S. aureus, E. coli and S. typhi [6]. Diarrhea remains a concern in developing countries despite development in public health and economic wealth. Presently, the drugs used in the treatment of diarrhea are associated with adverse effects such as GIT disturbances, skin rash, fever, eosinophilia synonym, dry mouth, etc. [7]. The World Health Organization (WHO) has presented a program to prevent diarrheal disease with traditional herbal medicines [8]. Medicinal plants exhibited antidiarrheal properties by controlling the gastrointestinal delay travel, suppress gut motility, increase water adsorption, or decrease electrolyte discharge [9]. Plants offer therapeutic effects because of the presence of substances like alkaloids, tannins, and essential oils which act by producing physiological activity on the human body [10]. Artemia nauplii or Brine shrimp used for the LC50 study of medicinal plants, which is a preliminary test for toxicity measurement [11, 12].
Hedychium coccineum (Zingiberaceae) is commonly known as the scarlet ginger lily, which used as an ornamental plant in native Asia. It is locally known as Aichhia and Mansila [13]. H. coccineum roots used in treating headaches and flowers pulped used in swollen body parts [14]. The Indian tribal people believe that wearing the flower behind the ear could be effective against the evil eye and disease [15]. It also reported being used as antipyretics and anti-inflammatory [13]. According to the reports, some essential oil compounds of H. coccineum found in Mauritius (East Africa) in rhizome part which is 44.4% of (E)-nerolidol, 24.2% of trans-sesquisabinene hydrate , α –Terpineol (0.6 %), α -fenchyl acetate (0.2 %) β-pinene (1.8%) and 2.4% of a-pinene [16]. There are no scientific report of traditional and pharmacological uses of H. coccineum. But a similar species named Hedychium coronarium available which used in various traditional uses such as inflammation, skin diseases, headache and rheumatic pain in Vietnam and China [17, 18].
Hence, the biological activity and phytochemical analysis not yet evaluated. So, the present study aimed to evaluate the in vivo antidiarrheal activity by Swiss albino mice and in vitro cytotoxicity by brine shrimp lethality assay and thrombolytic activity of methanolic extract of H. coccineum leaves.
MATERIALS AND METHODS
Chemicals and reagents
Loperamide (Square Pharmaceuticals Ltd. Dhaka, Bangladesh), castor oil (WELL’s Health Care, Madrid, Spain), methanol (Merck, Darmstadt, Germany) procured from the cited sources. Streptokinase (Beacon Pharmaceutical Ltd, Mymensingh, Bangladesh), vincristine sulfate (Sigma-Aldrich Co.) used in this study. All drugs and chemicals were of analytical grade.
Collection and preparation of extract
Leaves of H. coccineum collected from Fatikchari Upzilla in Chittagong (Chittagong Hill tracts Area) in February 2019 with the help of local guide Mr. Abul Kashem. The plant identified by botanist and taxonomist Dr. Shiekh Bokhtear Uddin, Professor, Department of Botany, University of Chittagong, Bangladesh. Freshly collected leaves cut into small pieces to make them suitable for grinding purposes. The leaves dried for ten days under shade and ground and finally dried in an oven at 45 °C for 24 hours. The materials were ground into a coarse powder with the help of grinder and macerated in 1.5 L methanol for 96 hours at room temperature with occasional shaking and stirring. Then the filtered through a cotton plug followed by Whatman filter paper [19]. The solvent was evaporated with water both at 40°C temperature to get viscous mass. The percentage of the yield of methanol extract H. coccineum leaves was 4.76%.
Experimental animals
Mice weighing range about 28-32 gm procured from the animal house of the Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh. All the animals familiarized themselves with the new environment for one week. During the experiment period, the animals kept in a well-ventilated animal house at 25 °C temperature. They supplied with standard pellets and fresh potable water. All the mice were kept within the cage in the animal house and maintained with a natural 12hrs light and dark cycle. The experimental animal handled according to international guidelines for the use and maintenance of experimental animals under the reference of Pharm/PND/161/31-2019 [20].
Antidiarrheal activity (In vivo)
Castor oil-induced diarrhea
Mice were fasted for 18 hours before the test with free access to water and divided into four groups (n=5). The mice were screened initially by giving 0.4 mL of castor oil and only those showing diarrhea selected for the experiment. The control group received vehicles only (distilled water containing 1% Tween-80), positive control received standard anti motility drug loperamide (5 mg/kg body weight) as oral suspension, test group received suspension of methanolic leaves extract of H. coccineum at the oral dose of 200 and 400 mg/kg body weight, respectively. After 1 hour of treatment, 0.4 mL of castor oil administered by oral gavage and placed in separate cages having adsorbent paper (blotting paper) at the bottom. The characteristics of diarrheal droppings (wet & dry faces) were noted every hour in four hours of study for each mouse. At the beginning of each hour, the old paper replaced with the new one [21].
Inhibition (%) = [(A-B)/A] × 100; where A = mean number of diarrheal feces of the control group; B = mean number of diarrheal feces of the treated group.
Gastrointestinal motility test by charcoal marker
Mice have treated same as the previously described method of Castor oil-induced diarrhea. After one hour of the oral administration, 1 mL of charcoal solution (10% charcoal, 5% gum acacia) given orally. Later, one hour’s mice sacrificed with a high dose of chloroform anesthesia. Measured the total length of the small intestine and the distance traveled by charcoal from the pylorus to cecum was measured [22].
Inhibition (%) = [(A-B)/A] × 100; where A = Distance travel by the charcoal control group (cm); B = Distance travel by the charcoal test groups group (cm).
Peristalsis index = (Distance travel by the charcoal meal / Total length of the small intestine) × 100
Brine shrimp lethality bioassay (In vitro)
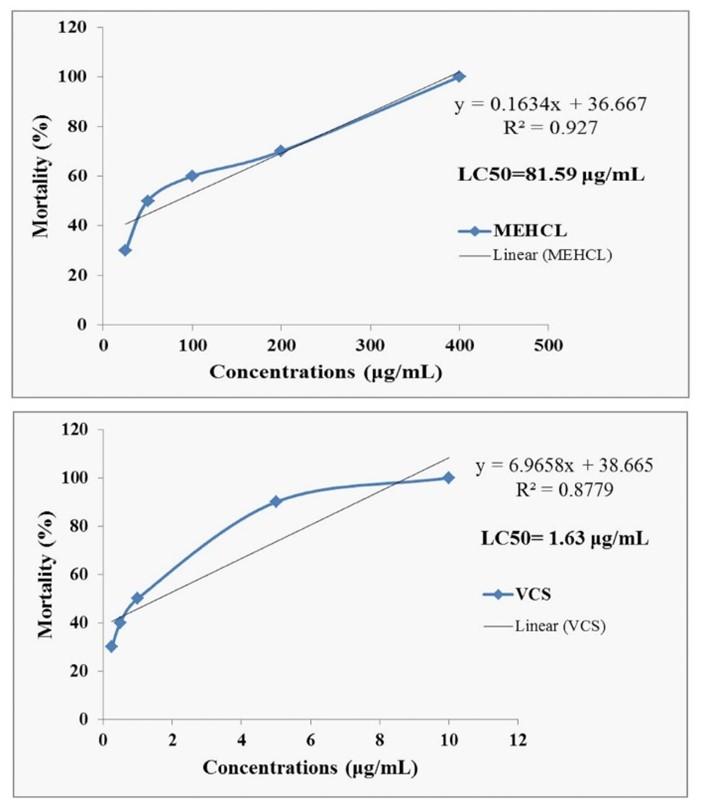
Brine shrimp lethality bioassay of cytotoxicity evaluated by using the using Artemia salina (shrimp eggs). In the artificial seawater (3.8% NaCl solution/l, w/v), the shrimp eggs hatched for 48 hours for maturing the shrimp called nauplii. The extract was dissolved in DMSO (50 µL in 5 mL solution) to prepare the test sample with artificial seawater (3.8% NaCl/L in tap water) to obtain the serially diluted concentrations of 25, 50, 100, 200 and 400 µg/mL. Vincristine sulfate used as a positive control as the preceding method in a serial concentration dilution 0.125, 0.25, 0.5, 1, 5 and 10 μg/mL. Each concentration contains ten nauplii. After 24 hours, all concentration inspected by an amplifying glass and the number of living and dead nauplii in each concentration was observed and recorded [23, 24].
% of mortality = (N1/N0) ×100
Where, N0= the number of nauplii taken; N1= the number of nauplii dead.
Thrombolytic activity (In vitro)
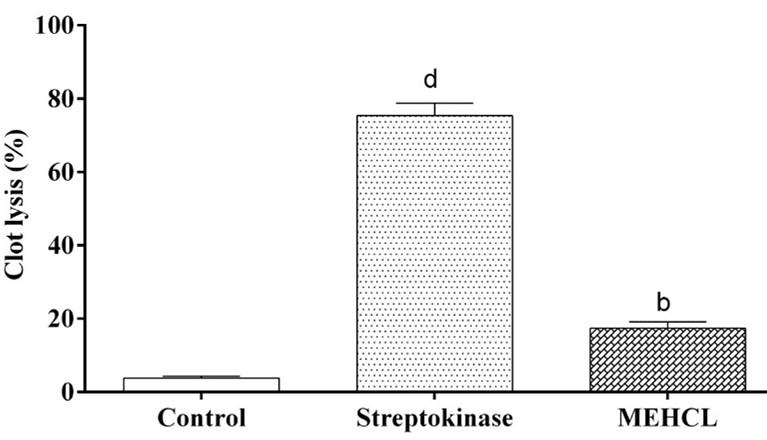
Thrombolytic activity test performed using the method described by Prasad et al. [25, 26]. As a stock solution, lyophilized streptokinase vial (1500000 IU) mixed adequately with 5 mL (30,000 IU) sterile distilled water. This suspension used as a stock from which 100 μL (30,000 IU) used for in vitro thrombolysis. A 3 mL of blood withdrawn from healthy volunteers (n=5, age: 20-23 years) without a history of anticoagulant therapy or an oral contraceptive. 0.5 mL per tube blood distributed to each previously weight eppendorf tubes and incubated at 37 °C for 45 minutes to form the clot. After the formation of the blood clot, removed the serum without disturbing the clot, and each eppendorf tube reweighed for calculating the clot weight. 100 µL extract (100 mg/10 mL) added to each tube having the pre-weighed clot. In similar manner, previously suspended streptokinase (100 µL) and 100 µL distilled water were added separately to each eppendorf while the streptokinase and distilled water used as the positive and negative control group. Incubation was done for 90 minutes at 37 °C and observed clot lysis. The released fluid was removed and reweighed the tube to calculate the difference in weight after clot disruption.
% of clot lysis = (weight of clot after remove of fluid/clot weight) × 100
Statistical analysis
The experimental results analysed by GraphPad Prism (version 7) software. Results represented in Mean ± Standard error mean (SEM) and statistical analysis carried by unpaired t-test of one-way ANOVA where P < 0.05 considered as statistically significant.
RESULTS
Antidiarrheal activity
Castor oil-induced diarrhea
The castor-oil induced diarrhea assay by methanolic extract of H. coccineum leaves (MEHCL) observed for four hours whereas a significant dose-dependent manner activity depicted (Table 1). Diarrheal episodes predominantly reduced by the positive control loperamide (5 mg/kg) in an extremely significant (P < 0.0001) manner (65.63%) while the MEHCL exhibited 42.71% and 53.12% by 200 and 400 mg/kg dose. In defecation phase, 400 mg/kg exhibited maximum inhibition (54.31%, P < 0.001) while the positive control loperamide (65.63%, P < 0.0001).
Table 1. The effect of methanolic extract of H. coccineum leaves on castor oil induced diarrhea in Swiss albino mice.
Castor oil-induced intestinal motility test (Charcoal marker)
The intestinal motility by castor oil-induced followed by charcoal marker exhibited an extremely significant (P < 0.0001) reduction in peristalsis movement for all doses of MEHCL when compared with the negative control. A maximum percentage of inhibition (41.98%, P < 0.0001) observed by 400 mg/kg dose followed by 23.67% in 200 mg/kg while the standard drug loperamide 48.09% as shown in Table 2.
Table 2. The effect of H. coccineum leaves extract with reference drug Loperamide on intestinal motility in mice by using charcoal as a marker.
Cytotoxic Assay
Brine Shrimp Lethality Bioassay
MEHCL showed different mortality rate at randomly selected concentration (25, 50, 100, 200 and 400 µg/mL). The mortality rate of brine shrimp nauplii found to be increasing with the increase of the concentration of the MEHCL. The LC50 values of MEHCL (81.59 μg/mL, R2 =0.927) whereas the LC50 values of Vincristine sulfate was 1.63 μg/mL (R2 = 0.8779). The results presented in Figure 1.
Thrombolytic activity
The addition of 100 µl SK, a positive control (30,000 I.U.) along with 90 minutes incubation at 37 °C, showed 75.35% clot lysis. Clots, when treated with 100 µl sterile 0.9% normal saline as a negative control, showed only negligible clot lysis 3.78%. The MEHCL exhibited a significant percentage of clot lysis (17.36%, P< 0.01). The results presented in Figure 2.
DISCUSSION
Diarrhea is generally the outcome of expanded electrolyte emission, altered intestinal motility, expanded luminal osmolarity and reduced electrolyte absorption which occurred by ricinoleic acid, an active component of castor oil [3, 27]. The discharge of ricinoleic acid from castor oil via lipase enzyme induces irritation in the intestinal mucosa. This irritation caused secretion of prostaglandin and nitric oxide, cyclic adenosine monophosphate, platelet-activating factor and tachykinins which are inflammatory mediators. The inflammatory mediators stimulate intestinal motility as well as electrolyte and water increase. This impact could happen as an outcome of enacting the G protein-coupled prostanoid receptor (EP3) on the smooth muscle cell of the intestine by ricinoleic acid [27, 28]. In our study, the MEHCL exhibited a significant reduction in the frequency of diarrhea and thus could be a potential source of phytochemicals that might inhibit the secretion of inflammatory mediators.
Intestinal motility test by charcoal marker used to determine the peristalsis movement of the intestine. The ricinoleic acid (bioactive components of castor oil) caused inflammation, irritation in the mucosa level of the intestine, which leads to diarrhea. Inflammation of intestine stimulates the release of prostaglandin resulting in intestinal motility as well as electrolyte and water increase [29]. The α–Terpineol has significant activity in blocking the PGE2 receptor to exhibit the antidiarrheal effect [30]. This plant contains α–Terpineol in its rhizome which might also present in the leaves as well. In our charcoal marker study, the MEHCL exhibited a significant inhibition in motility by inhibiting the synthesis of prostaglandin.
Brine shrimp lethality bioassay has widely utilized for screening of cytotoxic effects of plant extract [31]. Generally, the smaller the LC50, the higher the toxicity and vice versa. The value of LC50 over 1000 µg/mL considered to be non-toxic, ranging from 500 -1000 µg/mL is weakly toxic, moderately toxic for 100 – 500 µg/mL while less than 100 µg/mL is considered as highly toxic [32, 33]. The a-pinene and β-pinene reported having a highly toxic cytotoxic activity [34]. In our study, the MEHCL exhibited a toxic LC50 (81.59 μg/mL) whereas the vincristine sulfate (1.63 μg/mL). The mortality rate of brine shrimp nauplii found to be increasing with the increase of the concentration of the MEHCL. This plant contains a-pinene and β -pinene as an essential oil in its rhizome which might also present in the leaves as well. The observed cytotoxicity through brine shrimp lethality supports the earlier study on Hedychium coronarium which is different plant species of the Zingiberaceae family [35].
Most thrombolytic agents exert their beneficial effect by activating the enzyme plasminogen, which solubilizes the cross-linked fibrin mesh to restore blood flow over blocked blood vessels [36]. The lysis of clots, therefore, is useful for the treatment of clot-related disorders, including myocardial infarction, thromboembolic strokes, deep vein thrombosis, and pulmonary embolism, to clear a blocked artery that prevents permanent damage to the respective tissues [12, 37]. In our study, the MEHCL and streptokinase exhibited a significant percentage of clot lysis in comparison to negative control water. The increase in clot lysis by MEHCL compared to the controls demonstrates its potential use in clot-related disorders.
CONCLUSIONS
In our present study, the methanolic extract of H. coccineum leaves exhibited a significant antidiarrheal and thrombolytic activity with significant cytotoxicity (Figure 3). However, further advance study is required to predict the possible mechanism of H. coccineum leaves.
ACKNOWLEDGEMENT
Authors are very much thankful to the Department of Pharmacy, International Islamic University Chittagong, Bangladesh for research facilities and other logistic supports.
CONFLICTS OF INTEREST
Authors declared that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
FS and AMT together planned and designed the research. MAS and MNI arranged the whole facilities for the research and supervised the whole research. MAU, MAM and AU conducted the entire laboratory works with AMT and FS. FS and AMT imparted in study design and interpreted the results putting efforts on statistical analysis with MAS, MNI and TBE. FS, AMT and TBE participated in the manuscript draft and has thoroughly checked and revised the manuscript for necessary changes in format, grammar and English standard. All authors read and agreed on the final version of the manuscript.
References
- [1]Organization WHOJWH. Geneva: Diarrheal disease Fact Sheet No. 330. 2013.
- [2]Araújo TS, Costa DS, Sousa NA, Souza LK, de Araújo S, Oliveira AP, Sousa FBM, Silva DA, Barbosa AL, Leite JRSA. Antidiarrheal activity of cashew GUM, a complex heteropolysaccharide extracted from exudate of Anacardium occidentale L. in rodents. J Ethnopharmcol. 2015; 174: 299-307.
- [3]Uddin MMN, Zahan S, Islam M, Ahmed S, Mowla TE, Sofiqur MR, Sultan R, Emran TB. Evaluation of the anti-diarrheal activity of methanol extract and its fractions of Urena sinuata L. (Borss) leaves. J Appl Pharma Sci. 2016; 6: 056-60.
- [4]Pawlowski SW, Warren CA, Guerrant RJG. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterol. 2009; 136: 1874-86.
- [5]Perveen I, Hasan M, Masud M, Bhuiyan M, Rahman M. Irritable bowel syndrome in a Bangladeshi urban community: Prevalence and health care seeking pattern. Saudi J Gastroenterol. 2009; 15: 239-43.
- [6]Toyin YM, Khadijat OF, Saoban SS, Olakunle AT, Abraham BF, Luqman QA. Antidiarrheal activity of aqueous leaf extract of Ceratotheca sesamoides in rats. Bangladesh J Pharmacol. 2012; 7: 14-20.
- [7]Sarin RV, Narwal S, Bafna PA. Anti-diarrhoeal activity of aqueous extract of Ocimum kilimandscharicum. J Ethnopharmcol. 2013; 148: 223-8.
- [8]Atta AH, Mouneir SM. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. J Ethnopharmcol. 2004; 92: 303-9.
- [9]de Wet H, Nkwanyana MN, van Vuuren SF. Medicinal plants used for the treatment of diarrhoea in northern Maputaland, KwaZulu-Natal Province, South Africa. J Ethnopharmcol. 2010; 130: 284-9.
- [10]Olowa LF, Nuñeza OM. Brine shrimp lethality assay of the ethanolic extracts of three selected species of medicinal plants from Iligan City, Philippines. Int Res J Biological Sci. 2013; 2: 74-77.
- [11]Krishnaraju AV, Rao TV, Sundararaju D, Vanisree M, Tsay H-S, Subbaraju GV. Assessment of bioactivity of Indian medicinal plants using brine shrimp (Artemia salina) lethality assay. Int J Res Appl Sci Eng Technol. 2005; 3: 125-34.
- [12]Dash R, Emran TB, Paul A, Siddique MKU, Khan MA, Rahman MG, Sarwar MS, Uddin MMN. Effects of five Bangladeshi plant extracts on in vitro thrombolysis and cytotoxicity. Pharmacognosy Res. 2016; 8: 176-80.
- [13]Tushar, Basak S, Sarma GC, Rangan L. Ethnomedical uses of Zingiberaceous plants of Northeast India. J Ethnopharmacol. 2010; 132: 286-96.
- [14]Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology (5 Volume Set): CRC press; 2012.
- [15]Johnson T. CRC Ethnobotany Desk Reference. Boca Raton, Florida, USA: CRC Press Inc.; 1999.
- [16]Gurib-Fakim A, Maudarbaccus N, Leach D, Doimo L, Wohlmuth H. Essential oil composition of Zingiberaceae species from Mauritius. J Essent Oil Res. 2002; 14: 271-3.
- [17]Van Kiem P, Thuy NTK, Anh HLT, Nhiem NX, Van NJB. Chemical constituents of the rhizomes of Hedychium coronarium and their inhibitory effect on the pro-inflammatory cytokines production LPS-stimulated in bone marrow-derived dendritic cells. Bioorg Med Chem Lett. 2011; 21: 7460-5.
- [18]Lu Y, Zhong C, Wang L, Lu C, Li X, Wang PJ. Anti-inflammation activity and chemical composition of flower essential oil from Hedychium coronarium. Afr J Biotechnol. 2009; 8: 5373-377.
- [19]Vordermeier HM, Lowrie DB, Hewinson RG. Improved immunogenicity of DNA vaccination with mycobacterial HSP65 against bovine tuberculosis by protein boosting. Vet Microbiol. 2003; 93: 349-59.
- [20]Council NR. Guide for the care and use of laboratory animals: National Academies Press; 2010.
- [21]Shoba FG, Thomas MJ. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmcol. 2001; 76: 73-6.
- [22]Mekonnen B, Asrie AB, Wubneh ZB. Antidiarrheal activity of methanolic leaf extract of Justicia schimperiana. J Evid-Based Complement Altern Med. 2018; 2018: 10.
- [23]Kabir H, Shah M, Hossain MM, Kabir M, Rahman M, Hasanat A, Emran TB. Phytochemical screening, antioxidant, thrombolytic, α-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia K. Koch leaves. J Young Pharm. 2016; 8: 391-397.
- [24]Asaduzzaman M, Rana M, Hasan S, Hossain M, Das NJI. Cytotoxic (brine shrimp lethality bioassay) and antioxidant investigation of Barringtonia acutangula (L.). Int J Pharma Sci Res. 2015; 6: 1179-85.
- [25]Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thrombosis J. 2006; 4: 14.
- [26]Emran TB, Rahman MA, Uddin MMN, Rahman MM, Uddin MZ, Dash R, Layzu C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis. BMC Complement Altern Med. 2015; 15: 128.
- [27]Degu A, Engidawork E, Shibeshi WJBc, medicine a. Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del. (Euphorbiaceae) in mice model. BMC Complement Altern Med. 2016; 16: 379.
- [28]Jabri MA, Rtibi K, Ben‐Said A, Aouadhi C, Hosni K, Sakly M, Sebai H. Antidiarrhoeal, antimicrobial and antioxidant effects of myrtle berries (Myrtus communis L.) seeds extract. J Pharm Pharmacol. 2016; 68: 264-74.
- [29]Rahman M, Chowdhury M, Uddin A, Islam MT, Uddin ME, Sumi C. Evaluation of antidiarrheal activity of methanolic extract of Maranta arundinacea Linn. leaves. Adv Pharmacol Pharmaceutical Sci. 2015; 2015. Article ID: 257057.
- [30]dos Santos Negreiros P, da Costa DS, da Silva VG, de Carvalho Lima IB, Nunes DB, de Melo Sousa FB, Araújo TdSL, Medeiros JVR, dos Santos RF, Oliveira RdCM. Antidiarrheal activity of α-terpineol in mice. Biomed Pharmacother. 2019; 110: 631-40.
- [31]Ullah MO, Haque M, Urmi KF, Zulfiker AHM, Anita ES, Begum M, Hamid KJAP. Anti–bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac J Trop Biomed. 2013; 3: 1-7.
- [32]Nguta J, Mbaria J, Gakuya D, Gathumbi P, Kabasa J, Kiama S. Biological screening of Kenyan medicinal plants using Artemia salina (Artemiidae). Pharmacologyonline. 2011; 2: 458-78.
- [33]Ahmed S, Rakib A, Islam MA, Khanam BH, Faiz FB, Paul A, Chy MNU, Bhuiya NMA, Uddin MMN, Ullah SMA, Rahman A, Emran TB. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clin Phytosci. 2019; 5: 36.
- [34]Ramos EHS, Moraes MM, Nerys LLdA, Nascimento SC, Militão GCG, de Figueiredo RCBQ, da Câmara CAG, Silva TG. Chemical composition, leishmanicidal and cytotoxic activities of the essential oils from Mangifera indica L. var. Rosa and Espada. BioMed Res Int. 2014; 2014: Article ID: 734946.
- [35]Aziz MA, Habib MR, Karim MR. Antibacterial and cytotoxic activities of Hedychium coronarium. Res J Agric Biol Sci. 2009; 5: 969-72.
- [36]Bhattacharya S, Ploplis VA, Castellino FJ. Bacterial plasminogen receptors utilize host plasminogen system for effective invasion and dissemination. J Biomed Biotechnol. 2012; 2012: 19.
- [37]Rahman MA, Sultana R, Bin Emran T, Islam MS, Rahman MA, Chakma JS, Rashid H, Hasan CMM. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement Altern Med. 2013; 13: 25.