Tissue culture of Phalaenopsis: present status and future prospects
Abstract
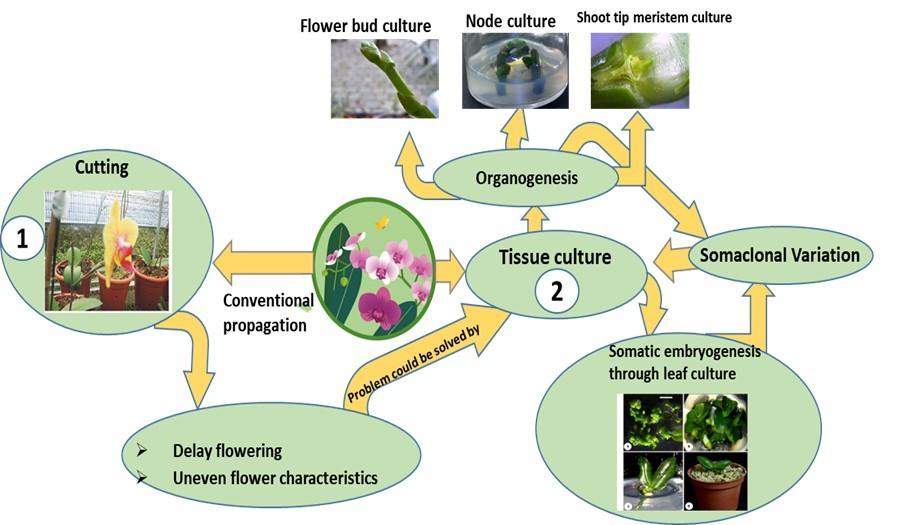
Phalaenopsis one of the popular cut-flower among the orchid species. The improvement/multiplication of this orchid is very difficult through conventional breeding due to delay flowering and uneven flower characteristics. Therefore, tissue culture techniques have been extensively used for improvement of Phalaenopsis by inducing and selecting somaclonal variants. However, it is difficult to get stable regenerations techniques of Phalaenopsis due to production of phenolic compounds, arising somaclonal variation in the culture and less recovery in the field of the regenerated plantlets. Improved and modified tissue culture techniques providing regeneration from various vegetative parts of plant are needed for industrialization and ex situ conservation of this valuable orchid. In this paper we have reviewed various in vitro propagation methods of Phalaenopsis culture which will be helpful for commercialization of this valuable orchid.
INTRODUCTION
Orchids considered as the most popular ornamental crop species in the world due to their unique use as cut flower and pot plants. Their ubiquitous beauty fascinated people since ancient times. Orchids are widely grown as ornamental cut flowers because of their exotic beauty and long shelf life [1]. Orchid cultivation is one of the most economically global trade nursery industries constituting a multi-billion dollar exchange among different countries [2, 3]. In contrast to world run-up, Bangladesh is in initial stage of orchid cultivation and starting orchid production commercially just few years ago along with the development of floriculture. In Bangladesh, BRAC, Proshika and Wonderland Toys etc. NGO’s are commercially planting orchids in a large scale. Orchids are bisexual plants and produced fruits after pollination and fertilization. They are normally produced large numbers of capsule, that are highly fragile and possess virtually no stored food material or endosperm [4].
Phalaenopsis (moth orchids) is one of the most popular among orchid species because of their specially beautiful and long-lasting flowers, and can cultivate quite easily in the artificial conditions [5-7]. The nomenclature of Phalaenopsis derived from Greek words phalaina, meaning moth, and opsis, meaning look-alike and the name describes the flowers apparently look like to flying moth [8]. In international flower market, these orchids have high economic value as cut flower. Today, Phalaenopsis are the most widely grown orchids. Statistical data from Netherlands show that Phalaenopsis market prospects increased from 5% to 66% in the year 1983 to 1994%, respectively [9]. As a monopodial plant Phalaenopsis are traditionally propagated by the cutting or division of off-shoots, however, these methods results low multiplication rate and hamper the growth of the mother plant, making them ineffective for large scale production. Therefore, their vegetative propagation is difficult and seedling characteristics are not uniform. It needs at least 3 years for flowering under greenhouse condition which is one of the vital problems in commercial production of Phalaenopsis. Therefore, tissue culture may be an efficient and alternative tool for propagation of this orchid species [10]. Thereafter, scientists in different corner in the world are trying their level best for commercial Phalaenopsis production through tissue culture technique (Figure 1). Inflorescence nodes of Phalaenopsis were induced to form plantlet in aseptic seed germination media by which laid the landmark of Phalaenopsis tissue culture [11]. Based on the findings of Rotor (1950), in vitro Phalaenopsis propagation and multiplication protocols have been developed by many researchers [12]. In this paper, we have tried to review the explant-based Phalaenopsis tissue culture starting from the pioneering works of Rotor (1950) to date and also the future perspectives of the tissue culture techniques for improvement of Phalaenopsis as well as in vitro conservation of the varietal purity of this species.
THE TRADE IN PHALAENOPSIS AND ITS CONTRIBUTION IN ECONOMY
Phalaenopsis are the second most important orchid marketed as both cut and potted flower. It is one of the most popular and economically important orchid genera at commercial scale production [13]. “Orchid growing has not fully achieved the transition from a hobby to an industry” stated by James Shoemaker in 1957. However, today, orchid growing is an international business and more than just an industry. Phalaenopsis are about 75% of all orchids sold [14]. Many countries like Germany, Netherlands, United States, China, Japan and Taiwan commercially grown the Phalaenopsis in large scale. Currently Phalaenopsis young plants production may have extended more than 300 million per year over the world [14]. Germany, Japan, United States, Netherlands and Taiwan commercially grown Phalaenopsis and Taiwan ranks tops in the world production [15]. In Taiwan Phalaenopsis export value increased from $8 million to $13 million in 2005 to 2006 [16], where worldwide turnover of Taiwanese Phalaenopsis raised from $27.5 million to $35.4 million from 2005 to 2006 [17]. Bangladesh is in very beginning stage of Phalaenopsis production. The Government of Bangladesh is now giving concern to meet up the local demand and can be participated in export market of Phalaenopsis.
PHALAENOPSIS REGENERATION THROUGH ORGANOGENESIS
Orchid could be propagated rapidly via protocorm like body (PLB) formation from explants rather than direct regeneration. However, recently PLB formation is considered as limited condition due to identify some crucial deleterious factors of orchid tissue culture. Therefore, scientists are seeking alternatives of the PLB formation in commercial orchid production. However, plant regeneration through the formation of PLB has been still practiced for mass propagation of monopodial orchid Phalaenopsis. Rotor, 1950 pioneered of vegetative propagation of Phaleonopsis gave the first documented report on micropropagation of Phaleonopsis using flower stalk cutting as explant [11]. Several reports have provided the indications regarding the flower-stalk cuttings would be very promising approach for clonal propagation of Phalaenopsis [11, 18-27]. Micropropagation of Phalaenopsis using flower stalk cuttings is the most widely used technique for mass propagation since the explants can be collected without damaging the mother plant [1]. In contrast , Tanaka et al. (1988) claimed that the flower stalk cuttings could not be used for large scale clonal propagation since the propagation rate is very low [28]. They suggested that the large scale propagation and multiplication of Phalaenopsis would be possible through the formation of PLB [22, 23, 29-34]. Murdad et al. 2006 reported protocorm is the unique structure for Phalaenopsis production and observed multiplication capability of trimmed and untrimmed protocorms using coconut water and activated charcoal on XER medium contain 20 gl-1 fructose [35]. Though Murdad et al. 2006 did not suggest that this protocol could be used for mass clonal propagation; they did stated that trimmed protocorm obtained from germinated seed is much better than untrimed one and trimmed protocorm cultured on coconut water and activated charcoal could be used for high frequency multiplication of Phalaenopsis gigantae. Chen et al. 2000 developed a reliable Phalaenopsis regeneration protocol using seed derived protocoms [36]. They used seed of Phalaenopsis nebula for the formation of protocorm in ½ MS basal medium and found that seed derived protocorm performed better for callus induction and subsequent plant regeneration from the induced callus. Whereas Tanaka and Sakanishi, 1985 recommended efficient seed germination of Phalaenopsis in MS medium through PLB formation using different leaf segment (distal, middle and proximal) from in vivo grown mature plant [37]. Park et al. 2002 reported an efficient in vitro Phalaenopsis regeneration protocol through PLB formation using flower stalk nodes derived leaf segments and recommended that modified hyponex medium is suitable for optimal number of PLBs [10]. They optimized the growth regulators combination, to obtain the highest regeneration of PLBs on½ MS medium with BA (88.8 µM) and NAA (5.4 µM) and for the first time they used the raft culture along with solid and liquid culture for proliferation of PLBs. Gonokbari, 2007gave an account for Phalaenopsis regeneration via protocorm formation through thin cell layer culture [38].They used ½ MS media with 2.0 mgl-1 BAP + 0.5 mgl-1 NAA along with coconut water and activated charcoal for PLB formation and used L-glutamine instead of plant hormone for shoot development from PLB. By using this method, they obtained large number of plantlet within a short period. Whereas, Tanaka, 1977 and Tanaka and Sakanishi, 1980 used both solid and liquid VW media with 20% coconut water for the proliferation of PLBs [39, 40]. MS medium used by Hass-Von, 1983 for proliferation and differentiation of PLBs. PLBs derived from ½ MS medium were cultured on solidified Hyponex medium (1 gl-1 6.5 N- 4.5 P- 19 N + 1 gl-120 N – 20 P – 20 K + 2 gl-1 peptone + 0.05% activated charcoal + 30 gl-1sucrose) for plantlets development [41]. They found that use of simple Hyponex medium during the proliferation and conversion of PLBs into plantlets was always advantageous. Among different liquid media VW liquid medium was effective for PLB multiplication [42]. Though Park et al. 2002 did not suggest any selective method that could be used on commercial scale vegetative propagation of orchid. Tokuhara and Mii, 1993 used New Dogashima Medium (NDM) instead of ½ MS medium for PLB formation containing 0.1 mgl-1 naphthaleneacetic acid (NAA) and 1mgl-1 6- benzylaminopurine (BAP) suggesting that their method could be used for vegetative propagation of Phalaenopsis and doritaenopsis on a commercial scale [43]. TDZ and auxins combination in culture medium found to be best for the induction of callus and PLBs from leaf of Phalaenopsis [44]. Maximum seed germination was observed in VW medium containing coconut water, with 1mgl-1 BAP and 2 mgl-1 kinetin. Callus and PLB were induced from the leaf of germinated plantlets on NDM medium containing TDZ, BAP and combination of TDZ and NAA. They found that TDZ in combination with NAA produce good quality and higher quantity PLBs than TDZ alone. Their result is contradictory with the findings of Soe et al. 2014. They found that PGR free MS medium was efficient for PLB formation [45]. In the propagation of Phalaenopsis Dora and doritaenopsis from inflorescence axis section, TDZ alone found to be more effective [46]. Arditti and Ernst, 1993 used modified MS medium with NAA and BA, Young et al. 2000 used MS medium with NAA and BA for PLB induction from leaf explants but these medium did not give any good result in case of Phalaenopsis gigantae so they used NDM medium for PLB induction [12]. They harvested plantlet after culturing the PLB and callus in hormone free NDM medium. Homma and Asahira, 1985 used inter-nodal section of flower stalk as explants to regenerate shoot of Phalaenopsis through PLB formation and PLB were produced from basal end of explants which touch the media [34]. Intermodal section of flower stalk was better than using flower stalk node and leaf culture in terms of duration of PLB formation and rate of contamination [34]. Among the different parts (tip, middle and basal) of PLB, the basal parts showed highest PLB formation in the PGR free medium [45]. Kobayashi et al. 1993 cultured protoplast derived from callus of lateral bud on flower stalks for regeneration of shoot and established a plant regeneration system from protoplasts culture in Phalaenopsis [47]. They pre-cultured the bud on P basal medium without coconut water and sucrose for 30 days for callus formation. The protoplasts were isolated from callus enzymetically and then cultured in the medium supplemented with 0.05-1.0 mgl-1 2,4-D and 10% cw. They found that 2,4-D was more important than CW for colony formation. Then the protoplast derived PLBs were placed on P basal regeneration medium (10% CW, 3% gelrite) for shoot regeneration. Tokuhara and Mii, 1993 developed an efficient PLB formation and subsequently plantlet regeneration method from PLB using shoot tip of flower stalk bud through cell suspension culture by selecting suitable carbohydrate source and concentration. They found that glucose produce the highest PLB than other carbohydrate sources used and lactose was not suitable for cell proliferation or PLB formation. Among the carbon sources, sorbitol was most suitable for plantlet initiation and development from PLB on Phalaenopsis regeneration [48]. They cultured lateral bud from young flower stalk in new Phalaenopsis medium (NP) with 10gl-1 sorbitol for callus induction. The PLBs were than cultured on NP medium supplanted with 20 gl-1 sucrose, 20 gl-1 maltose and 10gl-1 sorbitol and found that sucrose containing medium showed some necrosis while maltose and sorbitol medium have no necrosis and plantlet production was higher in sorbitol medium than sucrose and maltose medium.
Many researchers try to get regeneration without formation of PLB. Myint et al. 2001 have developed a rapid Phalaenopsis propagation technique through PLB (protocorm like body) formation using leaf as explant [49]. Plantlet was successfully regenerated via the adventitious bud without PLB formation from vegetative bud of flower stalk for avoiding somaclonal variation [50].They used Vacin and Went medium with 15% coconut water along with different concentration of TDZ and BAP and found that TDZ was more effective than BAP in stimulating the axillary buds for induction of shoots. Rotor, 1950 initiated in vitro Phalaenopsis cultures using flower stalks without disturbing the whole plant [11]. This technique found to be used extensively for mass propagation of Phalaenopsis. Dormant buds of the inflorescence were most advantageous among the other explants for in vitro propagation of Phalaenopsis; where Indole Acetyl amino Acids (IAA) used for the propagation of plantlets [33]. Flower stalk buds were cultured and achieved reproductive shoots from upper node and vegetative shoots from lower node [19, 22, 51]. Effect of bud position, temperature and BAP on the growth mode of bud studied in P. amabilis [51]. Lin, 1986 reported the influence of developmental stage and age of flower stalk on plantlet regeneration in Phalaenopsis and doritaenopsis and marked that the flower stalk with first flower and intermodal section near the tip of stalk have the highest regeneration capacity [52]. Kosir et al. 2004 used nodes with dormant bud of flower stalks for rapid shoot regeneration of Phalaenopsis [53]. They used six media with little difference in composition and found that none of the media was appropriate for mass generation and their result was contradictory with Arditii and Ernst (1993). They suggested that media with higher BAP and lower nitrogen content would be suitable in tissue culture and later for in vivo flower induction. BA was mandatory for floral bud induction where high nitrogen concentration inhibits the development of floral buds and shortening nine months growth period of Phalaenopsis for flowering [54]. Flower stalk culture is most frequently used as explants but it takes a long time to come out plantlets, leaf culture also need more time to produce protocorm and frequent release of phenolic compound is also a major problem. Plantlet regeneration through PLB formation is not easily reproducible [55]. Alternatively elongated stem node was used as explants for regeneration of plantlets by Duan et al. 1996 [55]. The elongated stems were cut into 4 section as top, second, third and basal node and placed on Hyponex medium supplemented with various concentration of BA. Shoots and multiple adventitious buds were produced after 70 days of culture and the highest number of shoots was obtained from third nodes and second nodes.
Phalaenopsis propagation through PLB formation was efficient but in many cases occurrence of somaclonal variation is a major problem for large scale production of plantlets. Chen et al. 1998 found considerable variation in flower colour and shape of Phalaenopsis “True Lady- B79-19” regenerated through tissue culture [56]. Tokuhara and Mii, 1998 also found somaclonal variation in flower and inflorescence axis of the micropropagated plants derived from flower stalk bud via protocorm like body formation of Phalaenopsis [57]. The variation ranges from 0 to 100% but maximum cultivars showed less than 10% somaclonal variation. Release of high phenolic compounds is another major problems of tissue culture of Phalaenopsis which is toxic for in vitro growing plantlets [58]. Use of bioreactor systems: continuous immersion system (air-lift type) and temporally immersion system could solve this problem. Temporary immersion bioreactor with activated charcoal filter attached was most suitable for multiplication of PLBs which was effective to remove phenolics. 83% PLBs regenerated into plantlets in 8 weeks and fresh weight of the plantlets and rooting percentage was also very high of the regenerated plantlets [58].
PHALAENOPSIS REGENERATION THROUGH SOMATIC EMBRYOGENESIS
Somatic embryogenesis has often been considered efficient techniques for plant regeneration and for obtaining transgenic plant. Currently somatic embryogenesis protocols have been successful studied in Phalaenopsis [59-62]. Successful regeneration of Phalaenopsis through somatic embryogenesis depends on many factors like source of explant, nutrient composition, the growth hormones and part of the explant taken, explant orientation etc. Kuo et al. 2005 reported plant regeneration using leaf explants through direct somatic embryogenesis after 20-30 days of culture on half-strength MS medium supplemented with BA and TDZ [61]. The frequency of embryogenesis is affected by explant orientation usually adaxial surfaces near wounded regions gave the highest embryogenic competency compared to other regions of explants though the authors have not clarify the reason for this. However Gow et al. 2008 found that the cut ends of leaf had the highest embryogenic competence than adaxial and abaxial sides in Phalaenopsis amabilis and Phalaenopsis nebula [63]. Cytokinin is effective for the somatic embryo induction. BA and TDZ has been reported to promoted embryogenesis mostly from the epidermal cell layers [61]. TDZ has also been reported to promote direct embryo formation from the epidermal cells and secondary embryogenesis from the leaf explants of Phalaenopsis amabilis [62] (Figure 2). Whereas NAA, 2,4-D highly retarded the somatic embryo formation from leaf explants of Phalaenopsis [61, 62].Concentrations of different plant growth regulators had effect on somatic embryogenesis from leaf explant of Phalaenopsis [64]. N6-benzyl adenine (6-BA) had better performance than adenine sulphate (Ad) in embryoid induction [64]. They reported that upper epidermis and single cell of mesophyll were the starting source of somatic embryos origination. Chen and Chang, 2004 reported TDZ promoted the formation of embryo from protocorm like bodies derived from seed; whereas NAA retarded the embryo formation of Phalaenopsis amabilis var. Formosa [65]. When protocorms derived from seed were cultured on ½MS medium without plant growth regulators except TDZ, 100% of the protocorms were produced embryos from the posterior regions. Regeneration of plantlets thorough somatic embryogenesis has also been achieved by Samson et al. 1998 [66]. They had used internodal flower stalk segment with an axillary bud to develop protocorms. They cultured the nodal cutting on Vacin-Went medium to develop vegetative shoots which were cultured on solid New Dogashima Medium (NDM1) supplemented with NAA and 4,4,4 tri-fluro-isopentenyl-adenine for the initiation of protocorm regeneration and histological study permit these protocorms as somatic embryo. They have recommended their methods for commercial propagation of Phalaenopsis. In the similar way, Tokuhara and Mii, 2001 developed a method for embryogenic calli; subsequently, plantlets from the calli using flower stalk bud by changing the sucrose concentration in NDM medium following liquid cell suspension culture [60]. Although Sajise and Sagawa, 1991 were first reported on embryogenic calli formation but they did not give any clear-cut protocol for callus induction [67]. However, Tokuhara and Mii, 2001 found that high sucrose concentration in media inhibit initial callus induction, but high sucrose plays vital role of callus proliferation, whenever callus being established in media [60]. Their proposed method could be efficiently utilized for the microprogation of Phalaenopsis despite about 10% somaclonal variations. Embryogenic cell suspension culture for the regeneration of plantlets from protoplast of Phalaenopsis wataboushi were followed using ½ NDM medium containing 0.06M sucrose, 0.44M sorbitol and 0.1g/l glutamine [68]. They established a plant regeneration protocol from protoplast without any plant growth regulators and coconut water as supplement. They used shoot tip for the induction of embryogenic calli following the protocol of Tokuhara and Mii, 2001, whereas one year old cells of suspension culture was used to isolate protoplast. As a carbohydrate source sorbitol (10gl-1 sorbitol in hormone free NDM medium with 0.3% gellun gum) considered most suitable for the regeneration of plantlets from PLBs and sucrose was most suitable for shoot development [68]. Ishii et al. 1998 has been reported that sucrose was effective for callus induction but somatic embryos were formed upon subculture in medium without sucrose indicated that the growth of monopodial orchid Phalaenopsis influenced by the sugar in medium [59]. Table 1 represents the brief scenario of tissue culture of Phalaenopsis.
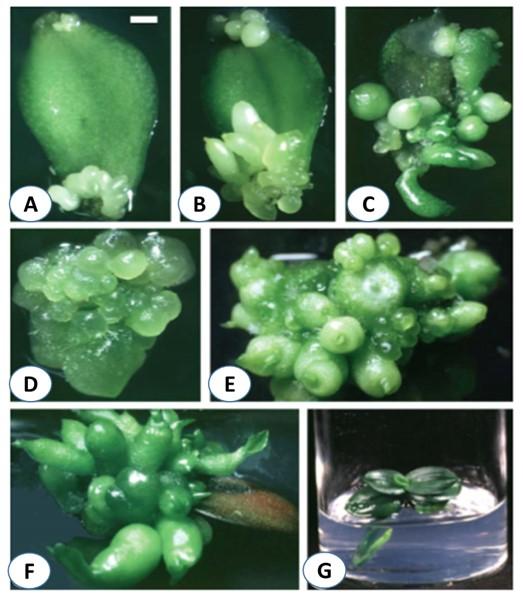
Table 1. Brief scenario of the success in micropropagation of Phalaenopsis orchid species using different explants and media by different researchers.
CONCLUSION AND FUTURE PROSPECTS OF PHALAENOPSISIS
Phalaenopsisis one of the most popular orchid and has immense economic value as ornamental cut flower. To date, the seed derived propagation of Phalaenopsis is very rapid and easy approach. Therefore, their uniform flower characteristics are one of the important criteria for commercialization. This could only possible by following tissue culture techniques despite very little somaclonal variation up to 10% within acceptable limit. Meanwhile, tissue culture method need less time to develop and maintain varietal purity compare to conventional breeding. In this paper we have tried to collect together most of the commercially important in vitro propagation of Phalaenopsis using different explants and different growth condition which will be helpful for rapid clonal propagation, industrial exploitation and also improvising the currently available method for in vitro mass propagation of this valuable orchid. The commercial demand of Phalaenopsis has been increasing day by day and Phalaenopsis production is now international in scope. Based upon the advanced tissue culture techniques new types might be developed with a compact growth habit, variegated foliage and uniform flower characteristics. Therefore, the Phalaenopsis trade might be increased both in volume and value throughout the world and possible to earn lots of foreign exchange by exporting the orchids.
ACKNOWLEDGEMENT
The authors acknowledge Department of Biotechnology, Patuakhali Science and Technology University, Bangladesh. The authors thankful to Dr. Chung, Mi-Young for her valuable efforts concerning this research.
AUTHOR CONTRIBUTIONS
Dr. Khadiza Khatun drafted the manuscript and revised the final draft. Dr. Ujjal Kumar nath revised the initial manuscript. Md. Shafikur Rahman helped to write the manuscript.
CONFLICTS OF INTEREST
Authors declared that they have no conflict of interest.
References
- [1]Chugh S, Guha S, Rao IU. Micropropagation of orchids: a review on the potential of different explants. Scientia Horticulturae, 2009; 122(4):507-520.
- [2]Hew C. Orchid cut-flower production in Singapore and neighboring ASEAN countries. American Orchid Society bulletin (USA), 1989; 887-897.
- [3]Alam M, Rashid M, Hossain M, Salam M, Rouf M. In vitro seed propagation of Dendrobium (Dendrobium transparens) orchid as influenced by different media. International Journal of Biotechnology (Pakistan), 2002; 1:111-115.
- [4]Mitra G. Studies on seeds, shoot-tips & stem-discs of an orchid grown in aseptic culture. Indian Journal of Experimental Biology, 1971; 79-85.
- [5]Guo WJ, Lin YZ, Lee N. Photosynthetic light requirements and effects of low irradiance and daylength on Phalaenopsis amabilis. Journal of the American Society for Horticultural Science, 2012; 137(6):465-472.
- [6]Lin MJ, Hsu BD. Photosynthetic plasticity of Phalaenopsis in response to different light environments. Journal of Plant Physiology, 2004; 161(11):1259-1268.
- [7]Liu YC, Tseng KM, Chen CC, Tsai YT, Liu CH, Chen WH, Wang HL. Warm-night temperature delays spike emergence and alters carbon pool metabolism in the stem and leaves of Phalaenopsis aphroide. Scientia Horticulturae, 2013; 161:198-203.
- [8]Mayr H. Orchid names and their meanings: Lubrecht & Cramer Limited, 1998.
- [9]Griesbach R. Potted Phalaenopsis orchid production: history, present status, and challenges for the future. HortTechnology, 2000; 10(3):429-429.
- [10]Park SY, Murthy HN, Paek KY. Rapid propagation of Phalaenopsis from floral stalk-derived leaves. In Vitro Cellular & Developmental Biology-Plant, 2002; 38(2):168-172.
- [11]Rotor JrG. A method of vegetative propagation of Phalaenopsis species and hybrids. In: Proceedings American Society for Horticultural Science, 1950.
- [12]Arditti J, Ernst R. Micropropagation of orchids. John Willey & Sons. Inc, NY, 1993.
- [13]Minh TN, Tuyen PT, Khang DT, Quan NV, Ha PTT, Quan NT, Andriana Y, Fan X, Van TM, Khanh TD. Potential use of plant waste from the moth orchid (Phalaenopsis Sogo Yukidian “V3”) as an antioxidant source. Foods, 2017; 6(10):85.
- [14]Griesbach R. Development of Phalaenopsis orchids for the mass-market. Trends in new crops and new uses ASHS Press, Alexandria, VA, 2002; 458-465.
- [15]Khoddamzadeh AA, Sinniah U, Kadir M, Kadzimin S, Mahmood M, Sreeramanan S. In vitro induction and proliferation of protocorm-like bodies (PLBs) from leaf segments of Phalaenopsis bellina (Rchb. f.) Christenson. Plant Growth Regulation, 2011; 65(2):381.
- [16]De L, Khan A, Kumar R, Medhi R. Orchid farming—a remunerative approach for farmers livelihood. International Journal of Science and Research, 2014; 3:468-471.
- [17]De LC, Pathak P, Rao A, Rajeevan P. 2 Global Orchid Industry. In: Commercial Orchids. Sciendo Migration, 2014; 13-19.
- [18]Sagawa Y, Niimoto D. Vegetative propagation of. In.: Phalaenopsis, 1960.
- [19]Urata U. The use of Ito-type vials for vegetative propagation of Phalaenopsis. American Orchid Society Bulletin, 1965; 34:410-413.
- [20]Scully R. Stem propagation of Phalaenopsis. American Orchid Society Bulletin, 1966; 35:40-42.
- [21]Intuwong O. Vegetative propagation of Phalaenopsis by flower stalk cuttings. Hawaii Orchid J, 1973; 1:13-18.
- [22]Koch L. Untersuchungen zur vegetativen Vermehrung bei Phalaenopsis in vitro. Technische Universität Hannover, 1974.
- [23]Koch L. Erbgleiche Vermehrung von Phalaenopsis in vitro. Gartenwelt, 1974; 74:482-484.
- [24]Reisinger DM. Clonal propagation of Phalaenopsis by means of flower-stalk node culture. American Orchid Society Bulletin, 1976; 45:45-52.
- [25]Zhongming Z, Linong L, Xiaona Y, Wangqiang Z, Wei L. Culture of flower-stalk buds a method for vegetative propagation of Phalaenopsis, 1977.
- [26]Flamee M, Boesman G. Clonal multiplication of Phalaenopsis-hybrids by means of sections of the flower stalk [ornamental plants]. Mededelingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent, 1977; 42: 1865-1868
- [27]Bouriquet R, Broly H, Legrand B. Clonal propagation of Phalaenopsis (Orchidaceae) by in vitro culture. Praeger, New York, 1982; 35-38.
- [28]Tanaka M, Kumura M, Goi M. Optimal conditions for shoot production from Phalaenopsis flower-stalk cuttings cultured in vitro. Scientia Horticulturae, 1988; 35(1-2):117-126.
- [29]Intuwong O, Sagawa Y. Clonal propagation of Phalaenopsis by shoot tip culture. American Orchid Society Bulletin,
- [30]Pieper W, Zimmer K. Clonal propagation of Phalaenopsis in vitro. In: Symposium on Production of Potted Plants and Cut Flowers, 1976; 64: 21-24.
- [31]Lay FFM. Studies on the tissue culture of orchids. I. Clonal propagation of Phalaenopsis by lateral buds from flower stems. Orchid Review,
- [32]Zimmer K, Pieper W. Clonal propagation of Phalaenopsis by excised buds. Orchid Review, 1978.
- [33]Griesbach RJ. The use of indoleacetylamino acids in the in vitro propagation of Phalaenopsis Scientia Horticulturae, 1983; 19(3-4):363-366.
- [34]Homma Y, Asahira T. New means of Phalaenopsis propagation with internodal sections of flower stalk. Journal of the Japanese Society for Horticultural Science, 1985; 54(3):379-387.
- [35]Murdad R, Hwa KS, Seng CK, Latip MA, Aziz ZA, Ripin R. High frequency multiplication of Phalaenopsis gigantea using trimmed bases protocorms technique. Scientia Horticulturae, 2006; 111(1):73-79.
- [36]Chen YC, Chang C, Chang WC. A reliable protocol for plant regeneration from callus culture of Phalaenopsis. In Vitro Cellular & Developmental Biology-Plant, 2000; 36(5):420-423.
- [37]Tanaka M, Sakanishi Y. Regenerative capacity of in vitro cultured leaf segments excised from mature Phalaenopsis Bulletin of the University of Osaka Prefecture Ser B, Agriculture and biology, 1985; (37):1-4.
- [38]Gonokbari S. Efficient micropropagation of Phalaenopsis amabilis (L.) Bl. cv.‘Cool Breeze’using inflorescence axis thin sections as explants. Propagation of Ornamental Plants, 2007; 7(1):9-15.
- [39]Tanaka M. Clonal propagation of Phalaenopsis by leaf tissue culture. American Orchid Soceity Bulletin, 1977; 46:733-737.
- [40]Tanaka M, Sakanishi Y. Clonal propagation of Phalaenopsis through tissue culture. In: Proc 9th World Orchid Conference, Bangkok, 1980; 215-221.
- [41]Hass-Von Schmude N. Klonale Massenvemehrung von Phalaenopsis. Die Orchidee, 1983; 34:242-248.
- [42]Park YS, Kakuta S, Kano A, Okabe M. Efficient propagation of protocorm-like bodies of Phalaenopsis in liquid medium. Plant Cell, Tissue and Organ Culture, 1996; 45(1):79-85.
- [43]Tokuhara K, Mii M. Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Reports, 1993; 13(1):7-11.
- [44]Niknejad A, Kadir M, Kadzimin S. In vitro plant regeneration from protocorms-like bodies (PLBs) and callus of Phalaenopsis gigantea (Epidendroideae: Orchidaceae). African Journal of Biotechnology, 2011; 10(56):11808-11816.
- [45]Soe KW, Myint KT, Naing AH, Kim CK. Optimization of efficient protocorm-like body (PLB) formation of Phalaenopsis and Dendrobium Current Research on Agriculture and Life Sciences, 2014; 32(4):179-183.
- [46]Ernst R. Effects of thidiazuron on in vitro propagation of Phalaenopsis and Doritaenopsis (Orchidaceae). Plant Cell, Tissue and Organ Culture, 1994; 39(3):273-275.
- [47]Kobayashi S, Kameya T, Ichihashi S. Plant regeneration from protoplasts derived from callus of Phalaenopsis. Plant tissue culture letters, 1993; 10(3):267-270.
- [48]Islam MO, Ichihashi S, Matsui S. Control of growth and development of protocorm like body derived from callus by carbon sources in Phalaenopsis. Plant Biotechnology, 1998; 15(4):183-187.
- [49]Myint KT, Chung MY, Chung JD, Kim CK. Propagation via in vitro culture of leaf tissue of Phalaenopsis Horticulture Environment and Biotechnology, 2001; 42(1):1-5.
- [50]Chen Y, Piluek C. Effects of thidiazuron and N 6-benzylaminopurine on shoot regeneration of Phalaenopsis. Plant Growth Regulation, 1995; 16(1):99-101.
- [51]Tanaka M, Sakanishi Y. Factors affecting the growth of in vitro cultured lateral buds from Phalaenopsis flower stalks. Scientia Horticulturae, 1978; 8(2):169-178.
- [52]Lin CC. In vitro culture of flower stalk internodes of Phalaenopsis and Doritaenopsis. Lindleyana, 1986, 1:158-163.
- [53]Košir P, Škof S, Luthar Z. Direct shoot regeneration from nodes of Phalaenopsis Acta agriculturae slovenica, 2004; 83(2):233-242.
- [54]Duan JX, Yazawa S. Floral induction and development in Phalaenopsis in vitro. Plant Cell, Tissue and Organ Culture, 1995; 43(1):71-74.
- [55]Duan JX, Chen H, Yazawa S. In vitro propagation of Phalaenopsis via culture of cytokinin-induced nodes. Journal of Plant Growth Regulation, 1996; 15(3):133-137.
- [56]Chen W, Chen T, Fu Y, Hsieh R, Chen W. Studies on somaclonal variation in Plant Cell Reports, 1998; 18(1-2):7-13.
- [57]Tokuhara K, Mii M. Somaclonal variations in flower and inflorescence axis in micropropagated plants through flower stalk bud culture of Phalaenopsis and Doritaenopsis. Plant Biotechnology, 1998; 15(1):23-28.
- [58]Young PS, Murthy H, Yoeup PK. Mass multiplication of protocorm-like bodies using bioreactor system and subsequent plant regeneration in Phalaenopsis. Plant Cell, Tissue and Organ Culture, 2000; 63(1):67-72.
- [59]Ishii Y, Takamura T, Goi M, Tanaka M. Callus induction and somatic embryogenesis of Plant Cell Reports, 1998; 17(6-7):446-450.
- [60]Tokuhara K, Mii M. Induction of embryogenic callus and cell suspension culture from shoot tips excised from flower stalk buds of Phalaenopsis (Orchidaceae). In Vitro Cellular & Developmental Biology-Plant, 2001; 37(4):457-461.
- [61]Kuo HL, Chen JT, Chang WC. Efficient plant regeneration through direct somatic embryogenesis from leaf explants of Phalaenopsis ‘Little Steve’. In Vitro Cellular & Developmental Biology-Plant, 2005; 41(4):453.
- [62]Chen J, Chang WC. Direct somatic embryogenesis and plant regeneration from leaf explants of Phalaenopsis amabilis. Biologia Plantarum, 2006; 50(2):169-173.
- [63]Gow WP, Chen JT, Chang WC. Influence of growth regulators on direct embryo formation from leaf explants of Phalaenopsis Acta Physiologiae Plantarum, 2008; 30(4):507.
- [64]Cui G, Hou X, Zhang Z, Zhang C, Hu N, LIU YC. Efficient somatic embryogenesis from leaf explants of Phalaenopsis in vitro culture and histological observations. Acta Hortic Sin, 2007; 34(2):431-436.
- [65]Chen JT, Chang WC. Induction of repetitive embryogenesis from seed-derived protocorms of Phalaenopsis amabilis var. formosa Shimadzu. In Vitro Cellular & Developmental Biology-Plant, 2004; 40(3):290.
- [66]Samson I, Hamama L, Letouzé R. The role of new synthetic cytokinins in the improvement of mass propagation of Phalaenopsis via protocorms regeneration. In: XIX International Symposium on Improvement of Ornamental Plants,1998; 508: 265-266.
- [67]Sajise JU, Sagawa Y. Regeneration of plantlets from callus and protoplasts of Phalaenopsis Malayan Orchid Bulletin, 1991; 5:23-28.
- [68]Shrestha B, Tokuhara K, Mii M. Plant regeneration from cell suspension-derived protoplasts of Phalaenopsis. Plant Cell Reports, 2007; 26(6):719.
- [69]Kubota K, Shimizu A, Kunitomo Y, Fujiki T. The study of clonal propagation methods in Phalaenopsis and related genus. Bulletin of the Yamanashi Agricultural Research Center (Japan),
- [70]Tokuhara K, Mii M. Highly-efficient somatic embryogenesis from cell suspension cultures of Phalaenopsis orchids by adjusting carbohydrate sources. In Vitro Cellular & Developmental Biology-Plant, 2003; 39(6):635.
- [71]Ling A, Yap C, Shaib JM, Vilasini P. Induction and morphogenesis of Phalaenopsis Journal of Tropical Agriculture and Food Science, 2007; 35(1):147.
- [72]Gow WP, Chen JT, Chang WC. Effects of genotype, light regime, explant position and orientation on direct somatic embryogenesis from leaf explants of Phalaenopsis Acta Physiologiae Plantarum, 2009; 31(2):363.
- [73]hepsithar C, Thongpukdee A, Kukieatdetsakul K. Enhancement of organic supplements and local fertilisers in culture medium on growth and development of Phalaenopsis ‘Silky Moon’protocorm. African Journal of Biotechnology, 2009; 8(18).
- [74]Myint KT, Kim CK, Chung MY, Park JS, Lim KB, Chung JD. Cyclic Micropropagation of Phalaenopsis using thin cross section of floral stalk-derived leaf. 원예과학기술지, 2009; 27(1):150-155.
- [75]Sinha P, Jahan M. Clonal propagation of Phalaenopsis amabilis (L.) BL. cv.’Golden Horizon’through In vitro culture of leaf segments. Bangladesh Journal of Scientific and Industrial Research, 2011; 46(2):163-168.
- [76]Rittirat S, Kongruk S, Te-chato S. Induction of protocorm-like bodies (PLBs) and plantlet regeneration from wounded protocorms of Phalaenopsis cornu-cervi (Breda) Blume & Rchb. f. Journal of Agricural Technology, 2012; 8:2397-2407.
- [77]Balilashaki K, Naderi R, Kalantari S, Soorni A. Micropropagation of Phalaenopsis amabilis Cool “Breeze” with using of flower stalk nodes and leaves of sterile obtained from node cultures. International Journal of Farming and Allied Sciences, 2014; 3(7):823-829.
- [78]Feng JH, Chen JT. A novel in vitro protocol for inducing direct somatic embryogenesis in Phalaenopsis aphrodite without taking explants. The Scientific World Journal, 2014; 2014.