Current knowledge on mechanisms involved in SARS-CoV-2 infection and kidney diseases
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is cause of a global pandemic which is demolishing global health and economy. SARS-CoV-2 infected patients are hospitalized with pneumonia where almost 20-30% of patients are led to kidney failure. The entry of SARS-CoV-2 into the systemic circulation leads to acute kidney injury (AKI) which may develop chronic kidney disease (CKD). In addition, patients who are diagnosed with AKI or CKD are at major risk of SARS-CoV-2 infection. Although a significant number of compounds have been proposed and the existing drugs have also been tested for repurposing, no specific therapy has been approved yet. SARS-CoV-2 invades human cells binding to the receptor of angiotensin-converting enzyme 2 (ACE2) via the receptor-binding domain. Cells that express ACE2 are susceptible to SARS-CoV-2 infection and the proportion of ACE2-positive cells in kidney proximal tubule is approximately 4%, indicating that SARS-CoV-2 might damage the kidney tubules leading to fatal kidney injury. Therefore, a better understanding of the potential mechanisms involved in SARS-CoV-2 infection-mediated kidney disease may unveil a novel therapeutic strategy against kidney diseases during COVID-19.
INTRODUCTION
Corona virus disease 2019 (COVID-19) pandemic is a serious concern worldwide [1]. On December 30, 2019, concentrated pneumonia cases were reported in the Hubei province of China, which were found to be linked with a seafood market in Wuhan. Chinese health authorities and the centers for disease control and prevention (CDC) announced that a novel coronavirus is causing pneumonia and international committee on taxonomy of viruses (ICTV) announced “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)” as the name of the new virus on 11 February 2020 and at the same time WHO also announced the name of the disease caused by the virus as coronavirus disease 19 (COVID-19) [2]. SARS-CoV-2 is a novel enveloped RNA beta coronavirus that causes pneumonia with fever, cough, and dyspnea [3]. In some cases, patients express non-respiratory symptoms and multiorgan failure such as kidney failure which indicates that SARS-CoV-2 might invade other organs such as heart, kidney, bladder, and esophagus [4]. In addition to respiratory organs, the expression of angiotensin-converting enzyme 2 (ACE2) protein has also been noticed in human kidneys [5]. The virus enters into a cell by its surface spike protein which binds to ACE2 receptor via its receptor-binding domain (RBD). SARS-CoV is proteolytically activated by human proteases but SARS-CoV-2 is preactivated by protease furin that reduces depending on human proteases for activation [6]. There is a pandemic spread of COVID-19 and the increased COVID-19 morbidity and mortality in patients with kidney disease [7, 8]. Although a significant number of compounds have been proposed and the existing drugs have also been tested for repurposing, no specific therapy has been approved yet. Therefore, it is imperative to understand the knowledge on the potential mechanisms involved in SARS-CoV-2 infection-mediated kidney disease may unveil a novel therapeutic strategy against kidney diseases during COVID-19.
SARS-CoV-2 INFECTION AND KIDNEY DISEASES
Like SARS-CoV-1 and MERS viruses, SARS-CoV-2 infections were expected to exhibit pneumonia-like syndromes such as diffused alveolar damage and acute respiratory failure [9]. Multiple organ like kidneys, gastrointestinal tract, liver has been reported during SARS-CoV-1 infection in 2003 [10] and recently in patients infected with SARS-CoV-2 [4, 11].
The heterogeneous disorders that affect the structure and function of the kidney are generally termed as chronic kidney disease (CKD). In CKD, kidney damage happens slowly and can’t filtrate blood the way they should do. Kidney damage (i.e, increase in albuminuria) or reduction of kidney function (ie, GFR< 60 mL/min per 1.73 m²) for 3 months or more, irrespective of clinical diagnosis may lead to development of CKD [12]. CKD is associated with an increased risk of both inpatient and outpatient pneumonia [13] where mortality rate in CKD patients appears to be 14–16 times higher [14]. Though only 0.7% of patients of 1099 cases had chronic kidney failure in Wuhan, China [15], during the early stages of the pandemic found that 2–4% of COVID-19 patients had chronic kidney failure [16]. Recent studies suggest that acute kidney injury (AKI) can contribute to the progression of CKD and end-stage renal disease (ESRD) [17, 18]. Endothelial injury, glomerular hypertension, interstitial fibrosis are the main causes of CKD associated with AKI. AKI is a severe symptom of the COVID-19 [4]. Among the AKI patients with SARS-COV-2 infection, 9% of cases are non-severe and 66% of cases are severe and among them, 49% patients died of due to severe AKI [7]. During AKI, the glomerular filtration rate (GFR) is suddenly reduced with retaining nitrogenous wastes. It also interrupts extracellular fluid volume, electrolytes, and homeostasis in the body [4]. COVID-19 patients who developed AKI had 5.3 mortality [19]. Intensive care unit (ICU) patients had increased creatinine (15.8% vs 4.1%) and BUN (26.3% vs 5.7%) [8]. In addition, autopsy studies suggest that patients with COVID-19 are consistent with direct viral infection of the kidney [20, 21].
Dialysis is required for both the AKI and CKD patients. Twenty-five percent of all CKD patients have a prior history of AKI [22]. Dialysis patients are more susceptible to upper respiratory tract infections as well as have a higher complication rate of these diseases [23]. Both AKI and CKD causes uremia, elevated the blood urea level, which is associated with inflammation and reduction of immune cell function [24]. This phenomenon can explain the susceptibility of CKD patients to SARS-CoV-2. As the number of COVID 19 patients increased, medical facilities and/or dialysis facilities for CKD patients became deficient. This might worsen the uremic condition and contribute to compromising the immunity of CKD patients leaving them more vulnerable to the infection. In addition, the most commonly found condition in CKD patients with SARS-COV-2 infection is type-2 diabetes and hypertension [25, 26]. Considering the seriousness of association of SARS-CoV-2 infection in kidney disease, it is necessary to find out the exact mechanisms involved in the pathogenesis to develop the potential therapy for the patients.
MECHANISM OF SARS-COV-2 IN KIDNEY DISEASES
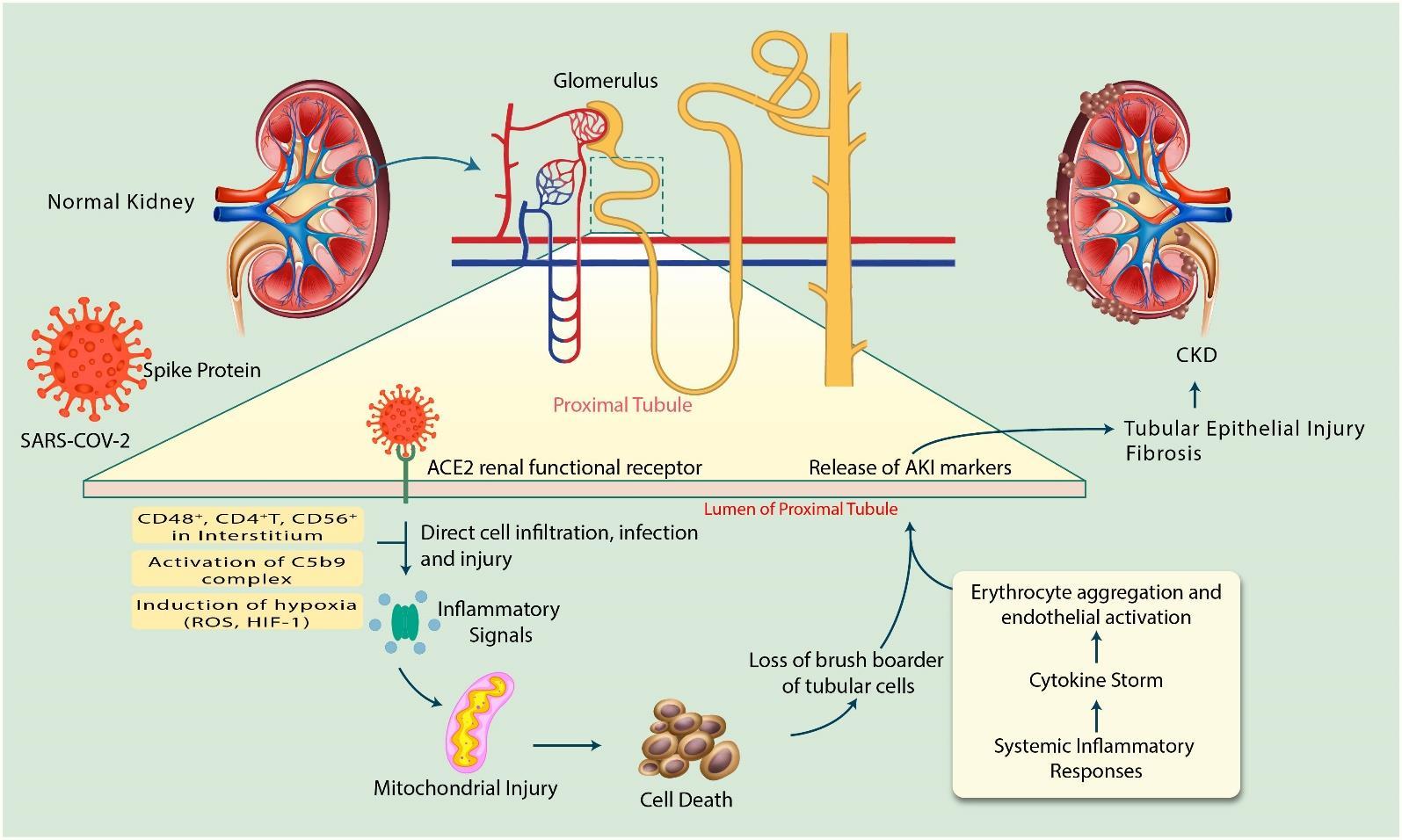
ACE2 expressing cells are the key targets for SARS-CoV-2 infections as they can allow virus entry, multiplication, spread, and pathogenesis [27]. On account of SARS-COV-2 infection, human kidney is a specific target for the virus since ACE2 expression is much higher in kidneys than the lungs [28]. Moreover, SARS-COV-2 has a great affinity for ACE2, consequently kidney may act as a reservoir for the virus [29]. As a matter of fact, the precise mechanism of SARS-CoV-2 to AKI or CKD is not well understood. Studies of patients with COVID-19 show that the direct viral infection of the kidney and expression of molecules that mediate viral entry may be cell type-specific [21]. In addition, AKI pathogenesis is multifaceted and associated mostly with kidney tubular cell injury [30-33]. Since the exact mechanism of kidney involvement in SARS-CoV-2 infection is unknown, here we have summarized the knowledge on mechanisms as below (Figure 1).
Recently, single-cell RNA-seq (scRNA-seq) was performed on the urinary system, specifically kidney and bladder [4]. Single-cell RNA-seq data demonstrate high expression of ACE2 in kidney proximal tubule and the bladder urothelial cells. The number of ACE2-positive cells in kidney proximal tubule and bladder urothelial cells was 4% and 2.4%, respectively. Therefore, the kidney should be considered as a high risk for potential COVID-19 infection since SARS-CoV-2 can invade non-respiratory organs [4]. In addition, corona virus (CoV)-infection increases inflammatory response by depletion of angiotensin (Ang) 1–7 and Ang 1–9 levels when ACE2 can cause kidney damage along with other organs [9]. Since glomeruli microscopy imaging showed normal morphologies in COVID-19 patients, it is postulated that kidney injury may be due to accumulation of immune complex of viral antigen or virus-induced effectors of immune system [34]. Proinflammatory cells like CD4+ T cells, CD56+ and CD68+ macrophages can cause direct cell infiltration and cell injury subsequently SARS-CoV-2 enter into proximal tubular cells [35]. SARS-CoV-2 can be detected in distal convoluted renal tubules and proximal straight tubular cells [5]. In addition, inflammatory cells such as CD68+ macrophages, CD4+T cells, CD56+ natural killer (NK) cells, and CD8+T cells were observed in the tubulointerstitium of the SARS-COV-2 infected tissue and hyperactivation of these inflammatory cells may enhance fibrosis, cause epithelial cell necrosis and bring about microvascular change [5, 36]. Further, the significant presence of viral RNA in urine was reported [37]. These observations suggest that SARS-CoV-2 infection-mediated inflammatory signals may lead to development of fibrosis in kidney disease.
The activation of C5b-9 complex, known as membrane attack complex observed in tubules or glomeruli of COVID-19 patients which causes renal parenchymal cells to release proinflammatory cytokines, ROS and profibrotic factors inducing acute tubular necrosis leading to kidney damage [38]. This study suggests that the development of AKI in SARS-COV-2 infection occurs also through C5b-9 expression and deposition since it is absent in normal kidney tissue [5]. In addition, cytokines release might exert indirect effects on renal tissue, such as hypoxia, shock, and rhabdomyolysis [16]. Insufficient blood flow-induces AKI increases HIF-1 and ROS leading to mitochondrial dysfunction [39]. In addition, a heatmap shows that SARS-CoV-2 infection downregulates mitochondrial processes such as electron transport chain and respiration [40].
Patients with severe SARS-CoV-2 infection requiring intensive care may suffer from cytokine storm syndrome (CSS) [41]. Cytokine storms can be caused due to excess production of immune cells [42] and mediators of pro-inflammatory and inflammatory cytokines [9, 41, 43]. Critical autoimmune inflammation and life-threatening edema were manifested in SARS-CoV-2 and SARS-CoV infected patients [41]. TH17 type responses, a marker of severe SARS-CoV-2 infection are induced by several cytokines, such as IL-17, IL-22, and TNFα [44]. A previous study demonstrates that cytokine release syndrome (CRS) or cytokine storm in the kidney induces AKI in SARS-COV-infected patients instead of active viral replication [45]. All of these occurred in AKI, subsequently develops kidney inflammatory response, microcirculatory dysfunction, mitochondrial injury, cell necrosis, and fibrosis leading to CKD or kidney damage [30, 31]. The synergistic effect of all of these factors may be connected to the increased incidence of AKI in COVID-19 patients along with state of dehydration, toxic tubular damage, and drug-induced nephrotoxicity [46].
AKI is directly linked to the progression of CKD and studies proved that AKI is also a cause of CKD [18]. Since COVID-19 is a recent epidemic and development of CKD required a period of months to a year, information from further studies are needed to understand the exact mechanisms in development of CKD in SARS-CoV-2 infected patients.
FUTURE DIRECTION AND CONCLUSION
SARS-CoV-2 infection has a fast expansion rate and can cause death to patients. COVID-19 patients who developed AKI or CKD are experiencing higher mortality. People of all ages are at risk of the SARS-CoV-2 virus infection. The virus can be exposed of by not only patients with clinical symptoms but also by them who are not showing any active clinical symptoms. There are no specific treatments or vaccines for this virus because the virus mutations could make them impractical. But by taking some effective steps [47-51], we can reduce the rate of death cases. SARS-CoV-2 infected patients who need dialysis should be instructed properly because of the inflexibility of diagnosis, and immunosuppression as well as use of antiviral drugs. Further, the medical workers should pay additional care to the dialysis patients infected by SARS-CoV-2 [52]. Since existence of SARS-CoV-2 in urine was revealed, urine tests might be potential diagnostic option for COVID-19, especially in developing countries.
In conclusion, the release/activation of AKI inducing factors such as mitochondrial injury, cell deaths, and loss of brush border in addition with the system inflammatory responses may induce tubular epithelial injury and fibrosis that ultimately may cause CKD during SARS-CoV-2 infection. Considering seriousness of kidney injury in SARS-CoV-2 infected patients, enormous effort must be paid by researchers to better understand the mechanism of action to identify new strategies to improve the therapeutic options for treating kidney disease in SARS-CoV-2 infected patients.
ACKNOWLEDGMENTS
This work acknowledges RP-Grant 2020 of Ewha Womans University, and the National Research Foundation (NRF grant No. 2020R1I1A1A01072879), Republic of Korea.
CONFLICT OF INTEREST
No conflict of interest from authors regarding the publication of this manuscript.
AUTHOR CONTRIBUTIONS
This work is a collaboration among all the authors. MJU designed outlines and drafted the manuscript. MHR, SJ, TAH, KAA, MK and MSO wrote the initial draft of the manuscript. MJU and AM reviewed the scientific contents described in the manuscript. All authors read and approved the final submitted version of the manuscript.
References
- [1]Sheam M, Syed S, Barman S, Hasan M, Paul D, Hasan R, et al. COVID-19: The catastrophe of our time. J Adv Biotechnol Exp Ther. 2020;3:1-13.
- [2]Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457-60.
- [3]Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355-62.
- [4]Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-92.
- [5]Diao B, Wang C, Wang R, Feng Z, Tan Y, Wang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Preprint from medRxiv. 2020.
- [6]Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727-34.
- [7]Durvasula R, Wellington T, McNamara E, Watnick S. COVID-19 and Kidney Failure in the Acute Care Setting: Our Experience From Seattle. Am J Kidney Dis. 2020;76:4-6.
- [8]Cao M, Zhang D, Wang Y, Lu Y, Peng L. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv In press 101101/2020030420030395. 2020.
- [9]Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- [10]Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977-85.
- [11]Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-13.
- [12]Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165-80.
- [13]Chou CY, Wang SM, Liang CC, Chang CT, Liu JH, Wang IK, et al. Risk of pneumonia among patients with chronic kidney disease in outpatient and inpatient settings: a nationwide population-based study. Medicine (Baltimore). 2014;93:e174.
- [14]Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120:1883-7.
- [15]Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-20.
- [16]Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829-38.
- [17]He L, Wei Q, Liu J, Yi M, Liu Y, Liu H, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017;92:1071-83.
- [18]Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58-66.
- [19]Anti-2019-nCoV Volunteers, Zhen L, Ming W, Jiwei Y, Yan J. Caution on kidney dysfunctions of 2019-nCoV patients. . MedRxiv In press 2020. https://doiorg/101101/2020020820021212.
- [20]Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-27.
- [21]Farkash EA, Wilson AM, Jentzen JM. Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. J Am Soc Nephrol. 2020;ASN.2020040432.
- [22]Ferenbach DA, Bonventre JV. Acute kidney injury and chronic kidney disease: From the laboratory to the clinic. Nephrol Ther. 2016;12 Suppl 1:S41-8.
- [23]Cohen-Hagai K, Rozenberg I, Korzets Z, Zitman-Gal T, Einbinder Y, Benchetrit S. Upper Respiratory Tract Infection among Dialysis Patients. Isr Med Assoc J. 2016;18:557-60.
- [24]Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255-65.
- [25]Soler MJ, Barrios C, Oliva R, Batlle D. Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep. 2008;10:410-4.
- [26]Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21.
- [27]Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-3.
- [28]Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397-406.
- [29]Perico L, Benigni A, Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron. 2020;144:213-21.
- [30]Doi K. Role of kidney injury in sepsis. J Intensive Care. 2016;4:17.
- [31]Togel F, Westenfelder C. Recent advances in the understanding of acute kidney injury. F1000Prime Rep. 2014;6:83.
- [32]Uddin MJ, Dorotea D, Pak ES, Ha H. Fyn Kinase: A Potential Therapeutic Target in Acute Kidney Injury. Biomol Ther (Seoul). 2020;28:213-21.
- [33]Uddin MJ, Pak ES, Ha H. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J Physiol Pharmacol. 2018;22:567-75.
- [34]Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698-705.
- [35]Khouchlaa A, Bouyahya A. COVID-19 nephropathy: probable mechanisms of kidney failure. J Nephropathol. 2020;9:e35.
- [36]Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366.
- [37]Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China. Am J Nephrol. 2020;51:343-8.
- [38]Rodriguez E, Gimeno J, Arias-Cabrales C, Barrios C, Redondo-Pachon D, Soler MJ, et al. Membrane Attack Complex and Factor H in Humans with Acute Kidney Injury. Kidney Blood Press Res. 2018;43:1655-65.
- [39]Pialoux V, Mounier R. Hypoxia-induced oxidative stress in health disorders. Oxid Med Cell Longev. 2012;2012:940121.
- [40]Singh K, Chen YC, Judy JT, Seifuddin F, Tunc I, Pirooznia M. Network Analysis and Transcriptome Profiling Identify Autophagic and Mitochondrial Dysfunctions in SARS-CoV-2 Infection. bioRxiv. 2020.
- [41]Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-4.
- [42]Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529-39.
- [43]Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102-8.
- [44]Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368-70.
- [45]Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16-32.
- [46]Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433.
- [47]Hannan MA, Rahman MA, Rahman MS, Sohag AAM, Das R, Hossian KS, et al. Fasting-mediated priming of host defense against SARS-CoV-2 infection: implication of autophagy and immune response. Immunol Lett. 2020;226:38-45.
- [48]Islam MN, Hossain MK, Sarker PP, Ferdous J, Hannan MA, Rahman MM, et al. Revisiting pharmacological potentials of Nigella sativa seed: a promising option for COVID-19 prevention and cure. Osfpreprints. 2020.
- [49]Hossain K, Hossain M, Moni A, Rahman M, Rahman U, Alam M, et al. Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. OSFpreprints, DOI: 1031219/osfio/w3hqu. 2020.
- [50]Hannan MA, Islam MN, Uddin MJ. Self-confidence as an immune-modifying psychotherapeutic intervention for COVID-19 patients and understanding of its connection to CNS-endocrine-immune axis. J Adv Biotechnol Exp Ther. 2020;3:14-7.
- [51]Farjana M, Moni A, Sohag A, Hasan A, Hannan M, Hossain M, et al. Repositioning vitamin C as a promising option for alleviating the complications associated with COVID-19. OSFpreprints, DOI:1031219/osfio/qamsw. 2020.
- [52]Xiao Y, Qian K, Luo Y, Chen S, Lu M, Wang G, et al. Severe acute respiratory syndrome coronavirus 2 infection in renal failure patients: a potential covert source of infection. Eur Urol. 2020;S0302-2838:30200-1.