Prevalence of multidrug resistance patterns of Escherichia coli from suspected urinary tract infection in Mymensingh city, Bangladesh
Abstract
Uropathogenic Escherichia coli (UPEC) is considered as one of the major bacteria causing urinary tract infection (UTI) affecting millions of people worldwide. UPEC rates of high resistance towards antibiotics have increased dramatically in recent years and made treatment difficult in Bangladesh. The study intended to determine the prevalence and antibiotic resistance pattern of E. coli from suspected urinary tract infections in Bangladeshi patients. A single cross-sectional retrospective observation was carried out in the Department of Microbiology at the Popular Diagnostic Centre, Mymensingh city, Bangladesh from August 2019 to August 2020. We collected data on urine culture from diagnostic reports of 4000 patients from which positive urine culture data were analyzed using SPSS software. During the study period, 453 positive urine cultures were identified from 4000 suspected UTI patients. Among them, 300 (66.2%) were female and 153 (33.8%) were male with their mean age of 45.50 (47.94 for male and 44.27 for female). According to the findings, Escherichia coli was the only uropathogenic bacterial species found in the patient’s urine culture. The highest antimicrobial resistance was seen among patients aged between 41 and 50 years. In an antimicrobial susceptibility test, 99% of isolates were resistant to at least one antibiotic, and 92% were multidrug-resistant (≥3 classes).
INTRODUCTION
Urinary tract infection (UTI) is the most severe bacterial infection constituting a common cause of emergency department visits, which results in getting antibiotics and hospitalization [1,2]. Increasing multidrug-resistant pathogens (especially resistant to the commonly used antimicrobials) is threatening our ability to treat these kinds of infections and increasingly being a concern [3, 4].
UTI is one of the major global health problems affecting almost 150 million people every year and responsible for about 8.1 million visits to health care providers each year [5-7]. The presence of microbial pathogens within the urinary tract is referred to as urinary tract infection, and the site of infection usually classifies it as the bladder (cystitis), kidney (pyelonephritis), or urine (bacteriuria). If the UTI is left untreated, it often results in serious complications leading to a rise in treatment costs and mortality [8]. The diagnosis of UTI must be based on positive urine culture. Urine serves as one of the important cultured ingredients for the growth of major pathogenic bacteria responsible for UTI [6]. UTI develops when a significant number of microorganisms (>105 cfu/ml) are found in urine [9]. UTI affects patients of all age groups and both sexes [10]. However, women are at higher risk of developing UTI than men. A short length of the urethra and its proximity to the anus as well as pregnancy and sexual activity, are some of the significant factors of UTI affecting women. The incidence of this infection in women is about 30% compared to only 1% in men [11-13].
According to the previous reports, Escherichia coli is the most common uropathogen causing UTI, which occurs in more than 80% of cases [10, 14, 15]. Primarily, based on the symptomatology and microbiological confirmations, acute UTI is treated with antibiotics. The excessive use and misuse of antimicrobials are considered significant factors in the rise of Multidrug-resistance (MDR) uropathogenic bacteria [15, 16]. The resistance pattern of uropathogens is changing significantly with time, specifically in developing countries, like Bangladesh [10]. The multidrug-resistant pathogens are making UTI treatment more difficult, increasing morbidity as well as mortality [5]. The emergence of multidrug-resistant E. coli causing UTIs is increasing, according to reports from the USA, Japan, China, India, Saudi Arabia, Brazil, and Nepal [17-22]. However, antibiotic resistance varies significantly between countries. Since most UTIs are treated based on observation early diagnosis, proper antimicrobial treatment is needed to reduce mortality and other complications [23]. Moreover, the prescribed antimicrobial agents should be determined based on the most likely pathogens and their expected resistance pattern in a geographic area. Regular monitoring is needed for the causative agents of UTI and their resistance patterns [24].
Antibiotic resistance is a consequence of bacterial adaptation to an antibiotic. Nowadays, a few antibiotics are used to treat a huge number of bacterial infections that favor the development of resistance [5]. Moreover, antibiotic resistance patterns may have significant variations in gender, age, and region. Therefore, a regional study from different periods is needed for a better understanding of the disease, its treatment, and prevention of complications. This study was aimed to analyze the MDR patterns to antibiotics of E. coli causing UTI among the people visiting a Diagnostic Centre at Mymensingh city in Bangladesh to facilitate better treatment and management of this common infectious disease.
MATERIALS AND METHODS
Study design and location
This study was conducted in Mymensingh city, Bangladesh, and the data were collected routinely with their written consent from the Microbiology Department of the Popular Diagnostic Centre from August 2019 to August 2020. The study was also approved by the ethical review committee of the Department of Biochemistry and Molecular Biology, Mawlana Bhashani Science and Technology University, Santosh, Tangail-1902, Bangladesh, with the certificate number MBSTU/BMB/TEST/2014/06(1).
Sample collection and laboratory analyses of bacterial culture
Urine samples were collected from sterile wide-mouthed urine cups. Each urine sample was then inoculated onto a Blood Agar base (Oxoid, Basingstoke, Hampshire, UK) with 10 % sheep blood, MacConkey agar (Oxoid), and HiCrome UTI Agar (HiMedia) in the Biosafety Cabinet Class II. The inoculated plates were then streaked using a calibrated loop and the plates were incubated at 37⁰c into an incubator for 18-24 hours for the growth of bacteria. The plates yielding colony counts more than or equal 100, 000 colonies/ml (≥105 / ml) of urine are regarded as significant bacterial growth [1]. Bacterial isolates were identified and characterized considering colony morphology, microscopic examination, and biochemical tests using Triple Sugar Iron (Oxoid) agar, Motility Indole Urea (Oxoid), and Simmons Citrate agar (Oxoid) following standard methods [5].
Antimicrobial susceptibility test (AST)
Antibiotic susceptibilities were performed for 453 E. coli isolates against 16 different antibiotic discs (Oxoid, UK) by standard disk diffusion technique as reported by Clinical and Laboratory Standards Institute (CLSI) using the Kirby-Bauer disk diffusion test on Mueller-Hinton agar [25, 26]. Different classes of antibiotics were used as follows: amoxicillin (penicillin), cephradine, ceftriaxone, and ceftazidime (cephalosporins); imipenem (carbapenems); erythromycin (macrolides), Co-trimoxazole (sulfonamides); moxifloxacin, nalidixic acid, ciprofloxacin, and levofloxacin (quinolones); gentamicin, netilmicin, and amikacin (aminoglycosides); nitrofurantoin (nitrofurantoin); aztreonam (monobactams). The antibiotic concentrations are as follows: amoxicillin (20 μg, Oxoid), cephradine (30 μg, Oxoid), ceftriaxone (30μg, Oxoid), ceftazidime (30 μg, Oxoid), imipenem (10 μg, Oxoid), erythromycin (15μg, Oxoid), Co-trimoxazole (25 μg, Oxoid), moxifloxacin (5μg, Oxoid), nalidixic acid (30μg, Oxoid), ciprofloxacin (5μg, Oxoid), levofloxacin (5μg, Oxoid), gentamicin (10μg, Oxoid), netilmicin (30μg, Oxoid), amikacin (30 μg, Oxoid), nitrofurantoin (300 μg, Oxoid) and aztreonam (30 μg, Oxoid). MDR was defined as resistance to at least three antimicrobial classes as suggested by Magiorakos et al. [27]. After measuring the zone of inhibition, results were determined compared to the Clinical and Laboratory Standards Institute (CLSI) guidelines [25]. The recommended reference strain of E. coli ATCC 25922 was used as a control to the antimicrobial test [26].
Statistical analysis
Statistical Package for Social Science (SPSS) version 24 was used for the data analysis (SPSS Inc., Chicago, IL, USA). Frequency distribution, Cross-tabulation, Chi-square test, Fisher exact test, Pie chart and Bar chart were applied for the statistical estimation of the variables.
RESULTS
Gender distribution of the total study subjects
Figure 1 shows the gender distribution of the total study subjects. A total of 4000 urine samples from different ages and sex of suspected UTI patients were processed to understand the resistance patterns of several antibiotics. During this study period, 11.32% (n=453) samples were found as growth positive; among them, 66.2% (n=300) were from female patients and 33.8% (n=153) from male patients with an approximate ratio of 2:1.
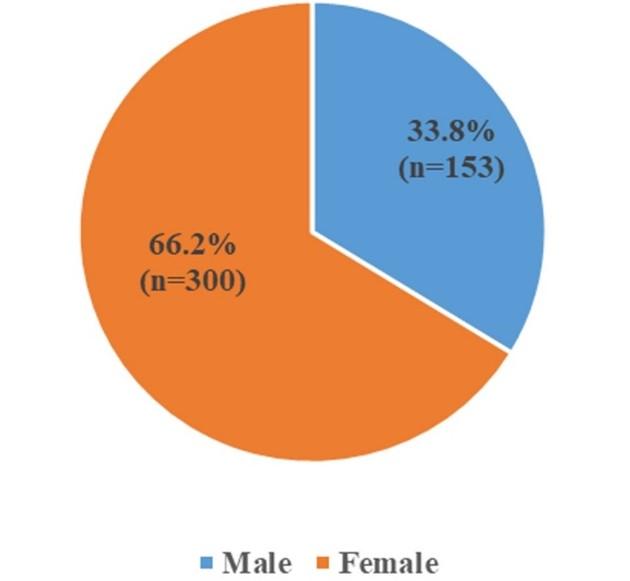
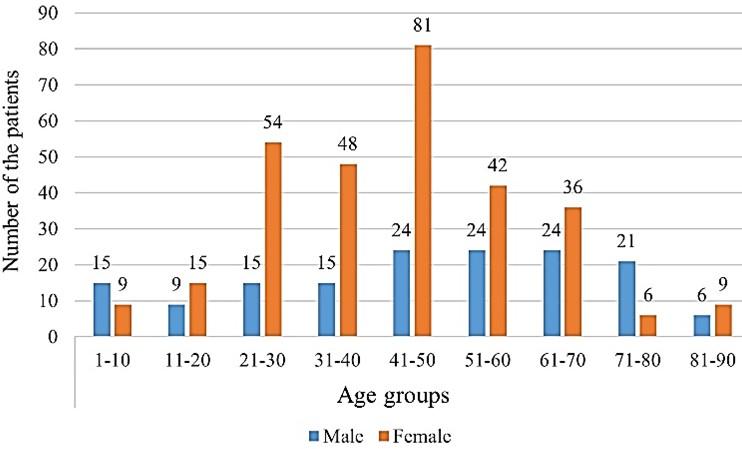
Gender distribution of total subjects according to age category
Figure 2 shows the gender distribution of the total UTI patients according to their age category. The mean age of the patients was 45.50 (47.94 for male and 44.27 for female). Based on the age of the patients, the highest rate of infection was observed in females except for the age groups of 1-10 and 71-80 years. In these age groups, males were found more infected than females on average. The maximum number of bacterial growths was observed in the age group of 41-50 years for both male and female.
Overall antibiotic susceptibility pattern of E. coli
Overall susceptibility profiles for Gram-negative bacteria with their percentages are summarized in Figure 3. In our study, E. coli was found to be the only causative agent in the sample of patients with urinary tract infection. In the AST, different types of antibiotic discs have been used.
Antibiotic susceptibility pattern of E. coli according to the age group of total patients
Table 1 shows the susceptibility pattern of E. coli against antibiotics according to patients ages group. Among 453 isolates, 99% of isolates were found resistant to at least one antibiotic, and 92% were found resistant to 3 or more classes of antibiotics, thus classified as MDR. E. coli was found to be most resistant to erythromycin (98%), followed by amoxicillin (89.4%), nalidixic acid (79.5%), co-trimoxazole (78.1%), cephradine (69.5%), ceftriaxone (60.9%), ciprofloxacin (59.6%), aztreonam (58.3%), levofloxacin (54.3%). Resistance of E. coli was less prevalent (<50%) to imipenem (7.3%), nitrofurantoin (15.9%), amikacin (18.5%), netilmicin (20.5%), gentamicin (28.5%), ceftazidime (46.4%). Those aged between 41 to 50 years shows the highest resistance pattern, followed by other age categories of the patients. Since E. coli is the only uropathogenic bacteria found in our study, the resistance profile of this Gram-negative bacteria is presented in more detail.
Table 1. Susceptibility pattern of E. coli against antibiotics according to patient age group.
Antibiotic susceptibility pattern of E. coli among male and female patients
In table 2, the distribution of the resistance pattern and its relation to gender are summarized. A statistically significant trend in the rate of antibiotic resistance according to the male and female was also assessed using chi-square and Fisher exact test for the following antibiotics: imipenem, gentamicin, nalidixic acid, levofloxacin, cephradine, aztreonam, erythromycin, ciprofloxacin, and nitrofurantoin. In our study, we found a significant difference in the resistance and sensitivity patterns of E coli. among the male and female patients. Here we observed E. coli was found to be the most resistant (99%) to erythromycin among the female patients, while the percentage was slightly less (96.1%) in the male patients. Furthermore, 91% of the female patients were resistant to amoxicillin, 77% to co-trimoxazole. In contrast, 86.3% male resistant to amoxicillin, 80.4% to co-trimoxazole. In case of sensitivity, imipenem had shown a significant sensitivity to E. coli, and the percentage was about 92% for females and 90.2% for males. Moreover, 85% of females and 78.4% of males were being sensitive to nitrofurantoin.
Table 2. Antibiotic susceptibility pattern of E. coli in male and female patients.
DISCUSSION
Antibiotic resistance is an alarming threat to our life due to its misuse and immoderate use. So, it is crucial for clinicians to be aware of the regional antibiotic resistance rates before prescribing any kind of antibiotics for UTI patients. Our study assessed the antibiotic resistance pattern of E. coli causing UTI among the people visiting the Popular Diagnostic Centre in Mymensingh city, Bangladesh. From our data, it is confirmed that E. coli is still the most common single microorganism causing UTI in patients of all age groups. MDR was observed, and surprisingly, E. coli being resistant to two-thirds of the antimicrobials of distinct classes. The second and third most common microorganisms differ significantly from region to region and from one study to another. A similar scenario is observed in our country as well [28-32]. According to our study, from 4000 urine samples, we found significant growth in 11.32% (n=453) clinical samples. The frequency of the isolated sample is close to the incidence reported by Ahmed, Avasarala (2008), and Begum et al. (2006), which was about 12.7% and 16.4%, respectively [33].
In the case of 11.32% (n=453) uropathogenic samples, the majority 66.2% (n=300) were from female and 33.8% (n=153) were from males. This value indicates that women are more likely to develop UTI than men, and at the age of 41 to 50, patients are more affected. Our results, in this regard, are significantly co-related to other studies [34-36]. Because of their anatomical and physiological changes, women are more prone to develop UTI than men. The reason is that the drier environment in the urethra of males prevents the optimal growth of bacteria. The antimicrobial activity of the prostate secretions and the long distance between the anus and urethra is one of the major factors that creates the differences in the prevalence of UTI between the two genders [37-39].
Repetitive unreasonable use of antibiotics changes the environment for the intestinal flora and leads to bacterial resistance [40]. In developing countries, antibiotic resistance has now become a public health concern, especially in countries like Bangladesh. Based on different studies in Bangladeshi population, most of the antibiotics here are found resistant to the uropathogen. Similar findings have also been reported in other parts of the world [40-41].
Our main prospect from this study was to focus on the uropathogenic E. coli and their resistance patterns to different groups of antibiotics commonly administered to treat the infection over the last years. Many factors may be involved, yet the abuse or misuse of antibiotics is the primary reason for the development of antibiotic resistance [11]. For first-line empirical treatment, the resistance pattern of antibiotics should not exceed 20% [43]. Our data reveals that most of the antibiotics have already crossed their safety limits and are unable to be treated as a first-line treatment for the suspected patients. Although the resistance patterns of netilmicin are about 20.5% (n=93), it is considered a sensitive antibiotic for Gram-negative bacteria.
In another study, it is noted that the level of antibiotic resistance profile should be 10% or less is suitable for empiric therapy [26]. If so, then our entire tested antibiotics are no longer appropriate for empiric management of UTI except imipenem. There is a significant discrepancy in these two studies. Therefore, a nationwide study is needed for the exact level of antibiotic resistance patterns because day by day, it has become an international threat to health. Not only uropathogenic but also other pathogens are now developing resistance to several antibiotics.
Sultana et al. (2018) [9] conducted a study in Dhaka, clearly shown that E. coli is 45% resistant against imipenem, 59% resistant to amikacin, 87% to gentamicin, and 66% to nitrofurantoin [9]. However, in our study, these antibiotics have shown a significant sensitivity to E. coli, which is conducted in Mymensingh city. There is an evident regional variation in the resistance pattern of antimicrobials to E. coli. It has been observed that the resistance of antimicrobial agents varies according to age, gender, and regional distribution. For the appropriate treatment, proper information is necessary regarding these resistance patterns of the current bacteria to give an effective antibiotic on time. In that case, health care providers should be aware of the resistance patterns of different uropathogenic bacteria.
We tried to explore the overall resistance patterns of several antibiotics against UTI infection in Mymensingh city, but it may not represent the whole situation in Bangladesh. So, a well-organized study is needed to find out the real scenarios of the multidrug-resistant uropathogenic bacteria among the general population of Bangladesh.
LIMITATIONS OF THE STUDY
The major limitation of the present study is the lack of clinical information to confirm whether urinary tract infections were in hospital or community-acquired and complicated or uncomplicated. The second thing was that the study might not represent the whole population in the Mymensingh city because only those who come to the diagnostic centre either by referral or as patients were included in this study. We emphasized those antibiotics that were only used for regional health purposes. Hence, our study was unable to talk about all the other types of antibiotics profiles in clinical practice. Also, we could not monitor the patient’s health patterns and information about the outcomes or any further diagnostic tests they performed.
CONCLUSION
MDR is widespread among uropathogenic bacteria. Due to high resistance to commonly used antibiotics, urinary tract infections, especially caused by E. coli, are now very difficult to treat empirically. Proper knowledge of local antimicrobial resistance patterns is essential for prescribing effective antibiotics. Our study may help in choosing a better treatment for urinary tract infection (UTI) patients in Bangladesh.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the authority of Popular Diagnostic Centre, Mymensingh, Bangladesh, for their wholehearted support of this work.
AUTHOR CONTRIBUTIONS
MJI conceived the development and designed the study. FAN, SAZ, and MRH contributed to the collection and assembly of the data. FAN, SA, TCS, RAJ contributed to the data entry. MMR and FAN contributed to the statistical analysis. FAN, SA, TCS, RAJ, and SAZ performed the first draft of the manuscript. MJI, KI, SS, AI, NA and FAN revised the manuscript and prepared the final draft of the manuscript.
CONFLICT OF INTEREST
Declaring no conflict of interest.
References
- [1]Kalaitzidou I, Ladomenou F, Athanasopoulos E, Anatoliotaki M, Vlachaki G. Susceptibility patterns of uropathogens identified in hospitalized children. Pediatr Int. 2019;61(3):246–251.
- [2]Mutters NT, Mampel A, Kropidlowski R, Biehler K, Günther F, Bălu I, et al. Treating urinary tract infections due to MDR E. coli with Isothiocyanates – a phytotherapeutic alternative to antibiotics? Fitoterapia. 2018;129:237–240.
- [3]Fagan M, Lindbæk M, Grude N, Reiso H, Romøren M, Skaare D, et al. Antibiotic resistance patterns of bacteria causing urinary tract infections in the elderly living in nursing homes versus the elderly living at home: an observational study. BMC Geriatr. 2015;15(1):98.
- [4]Mathai E, Grape M, Kronvall G. Integrons and multi-drug resistance among Escherichia coli causing community-acquired urinary tract infection in southern India. APMIS. 2004;112(3):159–164.
- [5]Alam J, Juliana FM, Rahimgir M, Hossain MN, Fatema B, Asaduzzaman M. Resistance Pattern of Ciprofloxacin against common Uropathogens in Selected Area of Dhaka city , Bangladesh. IOSR J Nurs Heal Sci. 2017;6(5):52–57.
- [6]Soni T, Kumar P, Prabhakar, Hussain S. Increasing Antibiotic Resistance in the Uropathogens. Asian J Pharm. 2019;13(1):1–4.
- [7]Bitew A, Molalign T, Chanie M. Species distribution and antibiotic susceptibility profile of bacterial uropathogens among patients complaining urinary tract infections. BMC Infect Dis. 2017;17(1):1–8.
- [8]Alzohairy M. Frequency and Antibiotic Susceptibility Pattern of Uro-Pathogens Isolated from Community and Hospital-Acquired Infections in Saudi Arabia – A Prospective Case Study. Br J Med Med Res. 2011;1(2):45–56.
- [9]Sultana R, Sarkar P, Khan J, Datta S. Prevalence of Multi-drug Resistance Pattern of Escherichia coli in Different Ages and Gender of Urinary Tract Infected Patients. Microbiol Res J Int. 2018;24(2):1–10.
- [10]Nahar A, Hasnat S, Akhter H, Begum N. Evaluation of antimicrobial resistance pattern of uropathogens in a tertiary care hospital in Dhaka city, Bangladesh. South East Asia J Public Heal. 2018;7(2):12–18.
- [11]Alam MJ, Asma R, Chowdury SS, Rahimgir M. Sensitivity pattern of cefotaxime against common uropathogens in vitro in Dhaka, Bangladesh. Drugs Ther Perspect. 2019;35(3):145–149.
- [12]Parveen R, Rahim I. Study of Bacterial Pathogens in Urinary Tract Infection and their Antimicrobial Sensitivity Pattern. Bangladesh J Infect Dis. 2018;4(2):40–44.
- [13]Lalhmangaihzuali FE, . Z, Varte Z, Laldinmawii G. Antibiotic resistance pattern of uropathogens in urinary tract infections in children at State Referral Hospital, Falkawn, Mizoram, India. Int J Contemp Pediatr. 2018;5(6):2108.
- [14]Córdoba G, Holm A, Hansen F, Hammerum AM, Bjerrum L. Prevalence of antimicrobial resistant Escherichia coli from patients with suspected urinary tract infection in primary care, Denmark. BMC Infect Dis. 2017;17(1):670.
- [15]Dehbanipour R, Rastaghi S, Sedighi M, Maleki N, Faghri J. High prevalence of multidrug-resistance uropathogenic Escherichia coli strains, Isfahan, Iran. J Nat Sci Biol Med. 2016;7(1):22.
- [16]Linhares I, Raposo T, Rodrigues A, Almeida A. Incidence and Diversity of Antimicrobial Multidrug Resistance Profiles of Uropathogenic Bacteria. Biomed Res Int. 2015;2015:1–11.
- [17]Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. Multidrug-Resistant Urinary Tract Isolates ofEscherichia coli: Prevalence and Patient Demographics in the United States in 2000. Antimicrob Agents Chemother. 2001;45(5):1402–1406.
- [18]Niranjan V, Malini A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J Med Res. 2014;139(6):945–948.
- [19]Ishikawa K, Hayakawa S, Miyakawa S, Kusaka M, Shiroki R, Hoshinaga K. Survey of the susceptibility of urinary isolates to antibacterial agents in 2003. J Infect Chemother. 2005;11(1):44–47.
- [20]Ho P-L, Wong RCW, Yip K-S, Loke S-L, Leung MST, Mak GC, et al. Antimicrobial resistance in Escherichia coli outpatient urinary isolates from women: emerging multidrug resistance phenotypes. Diagn Microbiol Infect Dis. 2007;59(4):439–445.
- [21]Rafay AM, Nsanze HN. Multi-drug resistance of Escherichia coli from the urinary tract. Saudi Med J. 2003;24(3):261–264.
- [22]Kumari N, Ghimire G, Magar JKG, Mohapatra TM, Rai A. Antibiogram pattern of isolates from UTI cases in Eastern part of Nepal. Nepal Med Coll J. 2005;7(2):116–118.
- [23]Gunduz S, Uludağ Altun H. Antibiotic resistance patterns of urinary tract pathogens in Turkish children. Glob Heal Res Policy. 2018;3(1):10.
- [24]Ramírez-Castillo FY, Moreno-Flores AC, Avelar-González FJ, Márquez-Díaz F, Harel J, Guerrero-Barrera AL. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: cross-sectional study. Ann Clin Microbiol Antimicrob. 2018;17(1):34.
- [25]Institut C and LS. Clinical and Laboratory Standards Institute. In: CLSI document M07-A9. 2019. p. 604.
- [26]Abejew AA, Denboba AA, Mekonnen AG. Prevalence and antibiotic resistance pattern of urinary tract bacterial infections in Dessie area, North-East Ethiopia. BMC Res Notes. 2014;7(1):1–7.
- [27]Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281.
- [28]Yüksel S, Öztürk B, Kavaz A, Özçakar ZB, Acar B, Güriz H, et al. Antibiotic resistance of urinary tract pathogens and evaluation of empirical treatment in Turkish children with urinary tract infections. Int J Antimicrob Agents. 2006;28(5):413–416.
- [29]Ipek IO, Bozaykut A, Arman DC, Sezer RG. Antimicrobial resistance patterns of uropathogens among children in Istanbul, Turkey. Southeast Asian J Trop Med Public Health. 2011;42(2):355–362.
- [30]Yolbaş I, Tekin R, Kelekci S, Tekin A, Okur MH, Ece A, et al. Community-acquired urinary tract infections in children: pathogens, antibiotic susceptibility and seasonal changes. Eur Rev Med Pharmacol Sci. 2013;17(7):971–976.
- [31]Bean DC, Krahe D, Wareham DW. Antimicrobial resistance in community and nosocomial Escherichia coli urinary tract isolates, London 2005 – 2006. Ann Clin Microbiol Antimicrob. 2008;7(1):13.
- [32]Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J N M C Hospital Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6(1):4.
- [33]Ahmed SM, Avasarala AK. Urinary tract infection (UTI) among adolescent girls in rural Karimnagar district. AP-K.A.P. Indian J. 2009;40(1):1–2.
- [34]Deshpande KD, Pichare AP, Suryawanshi NM, Davane MS, Article O. Antibiogram of Gram Negative Uropathogens in Hospitalized. Int J Recent Trends Sci Technol. 2011;1(2):56–60.
- [35]Gn M, Prakash R, Annam V, Shetty K, Res. IJBM. Changing trends in the spectrum of antimicrobial drug resistance pattern of uropathogens isolated from hospitals and community patients with urinary tract infections in tumkur and bangalore. 2011;2(2):504–507.
- [36]Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(90001):1–7.
- [37]Kibret M, Abera B. Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia. Asian Pac J Trop Biomed. 2014;4(2):164–168.
- [38]State N, Durowade K. Prevalence of urinary tract infections (UTI) among patients attending Dalhatu Araf Specialist Hospital ,. J Med Med Sci. 2009;1(5):163–167.
- [39]Prasada S, Bhat A, Bhat S, Shenoy Mulki S, Tulasidas S. Changing antibiotic susceptibility pattern in uropathogenic Escherichia coli over a period of 5 years in a tertiary care center. Infect Drug Resist. 2019;Volume 12:1439–1443.
- [40]Costa D, Vinué L, Poeta P, Coelho AC, Matos M, Sáenz Y, et al. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli isolates in faecal samples of broilers. Vet Microbiol. 2009;138(3–4):339–344.
- [41]Alemu A, Moges F, Shiferaw Y, Tafess K, Kassu A, Anagaw B, et al. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes. 2012;5(1):197.
- [42]Assefa A, Asrat D, Woldeamanuel Y, G/Hiwot Y, Abdella A, Melesse T. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at Tikur Anbessa Specialized Hospital Addis Ababa, Ethiopia. Ethiop Med J. 2008;46(3):227—235.
- [43]Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–120.