Exercise and oral melatonin attenuate anxiety and depression like behavior in type 2 diabetic rats
Abstract
This study was performed to evaluate the therapeutic efficacy of exercise and oral melatonin on metabolic syndrome (MS), anxiety and depression-like behavior (ADB) in type 2 diabetes mellitus (T2DM) model in rat. Rats were allocated into five groups: non diabetes group, diabetes group, three treated group; diabetes rats disciplined with swimming exercise (40 min, 5 days per week) or oral melatonin (10 mg/kg bwt per day at 19.00 PM) alone or with combination. Exercise and oral melatonin significantly attenuated MS evidenced by improvement of hyperglycemia, insulin resistance, dyslipidemia, hyperleptinemia, and hypoadiponectinemia level in comparison with diabetes group. The ADB also markedly improved in exercise and oral melatonin treated rats as represented by decreased anxiety index, increased open arm entries and time spent in elevated plus maze, and reduced freezing behavior, increased entries and time spent in center in open field test. To know underlying molecular mechanisms, hippocampus tissue was analyzed. Interestingly, exercise combined with oral melatonin synergistically reduced serum corticosterone and hippocampus tissue level inflammatory cytokines and improved ATP level. Furthermore, this combination up-regulated the expression of brain-derived neurotrophic factor (BDNF), peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) and mitochondrial biogenesis related proteins, glucose transporter type 4 (GLUT4) in hippocampus tissue. Exercise and oral melatonin synergistically attenuated ADB in T2DM rats by attenuation of MS, neuroinflammation and normalizing corticosterone level via up-regulation of PGC-1α, mitochondrial biogenesis, BDNF, GLUT4, expression and ATP level. Thus, this treatment combination can be a promising tool in the management of MS, anxiety and depression in T2DM patients.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is known as complex metabolic disease comorbidities demonstrated mainly by high blood glucose and insulin resistance (IR) caused by abnormal secretion of insulin or alteration of cellular up regulation [1]. It induces for the imbalance between energy consumption and expenditure. The incidence of T2DM is rapidly increasing worldwide due to habits in modern socio-economic and technology based lifestyle which has reduced the laborious activities or increased physical inactivity [1, 2]. Currently, 425 million adults have diabetes and 1 in 2 remains undiagnosed and by 2030 it is anticipated that about 552 million adults worldwide will be affected by this disease [3]. Diabetes and its hyperglycemia exert a remarkable threat to health across the globe due to its severe impacts on the microvascular systems which lead to dysfunction of multiple organs especially the kidneys, heart, eyes, brain, nerves, blood vessels [4]. The occurrence of neurohormonal disorders and neuropsychiatric symptoms including depression and cognitive dysfunction are higher in T2DM [1, 5, 6]. Altogether, these symptoms and complications in diabetic patient significantly deteriorate the quality of life. Moreover, T2DM and depression are two major issues of morbidity and mortality, currently more than 9% and 5% respectively, of the global population [6]. One fourth patients with T2DM suffer form of depression, five-times severe than recorded in the general public [7]. Depression disease comorbid with symptoms of anxiety between 50 and 75% with many common factors, including hypothalamic-pituitary-adrenal axis dysregulation, activation of inflammatory sequence, and impact on functional imbalance [6]. Tiller JW, [8] reported that 90% of patients with anxiety disorder have depression and about 85% of patients with depression have significant anxiety. Unsurprisingly, this comorbid have a poorer prognosis, with greater severity, and prolong treatment period [9], it might be severe when combined with diabetes.
Physical exercise is believed to have many beneficial effects on the incidence of cardiovascular diseases, obesity, and diabetes; conversely, a sedentary lifestyle has been recognized as a risk factor for many diseases [10, 11]. Acute exercise induces transient oxidative stress but regular controlled exercise improves metabolic diseases and antioxidant activities [1, 12-14]. Since it is frequently recommended as an important tool to promote optimal health and life expectancy. Melatonin is primly produced by the pineal gland regulates many biological functions including cardiovascular and immune functions, neuroendocrine, circadian rhythms, direct or indirect powerful anti-oxidative activities, anti-inflammatory effects and enhancing the capacity of mitochondrial activity [1, 15, 16]. Many studies reported therapeutic efficacy of melatonin against diabetes [15, 17, 18]. However, a few studies [1, 19] have found that deals with the joint actions of exercise and oral melatonin, but still many mechanisms remain to be elucidated.
Therefore, the purpose of this study was to evaluate the combined effect of oral melatonin and exercise on metabolic syndrome, depression in T2DM rat model and to gain insight into the underlying molecular mechanisms.
MATERIALS AND METHODS
Study design
Male white Sprague-Dawley rats (Orient Bio, Gyeonggi-do, Korea) average body weight was 219±1 g. The 75 rats were equally allocated into five groups: non-diabetes normal control group (NC), T2DM control group (DM), diabetes rats were disciplined with exercise (DME), diabetes rats treated with oral melatonin (DMM) and diabetes rats treated with oral melatonin and exercise (DMME) groups. Melatonin was supplied orally 10 mg/kg body weight/day at 19.00 PM and 40 min swimming per day, 5 days per week were disciplined after diabetes confirmation. Fresh melatonin (Sigma Chemical Co, St Louis, MO, USA) administered at the dose of 10 mg/kg body weight [1, 20, 21] by oral gavage at 19.00 PM. Swimming exercise was disciplined 40 min/day and 5 days/week. Swimming exercise regimentation was done in a specially designed temperature controlled swimming pool as described previously [1]. The three animals together at a time were enforced for swimming 40 minutes at a time. T2DM was induced by feeding 60% high fat diet (45 days, then streptozotocin (40 mg/kg body) and nicotinamide (200 mg/kg body) IP injection followed by method as described previously [1, 22]. The fasting blood glucose (FBG) levels were measured and rats with 12 mmol/l were considered T2DM [1, 22]. The study protocol was approved by laboratory animal ethics committee of KNOTUS Co., Ltd, Korea (Registration number: 16-KE-100-2).
Elevated plus-maze
Typical anxiety-like behaviors of rodents considered in elevated plus-maze by reducing number of entries and less time spent in the open arms as well as increased amount of time in the closed arms. This test was performed as described elsewhere [23]. Briefly, the cross shaped elevated plus maze consisted of total four arms and one center square, two open arms (10 x 50 cm) and two closed arms (10 x 50 x 40 cm) interconnected by a central square (10 x 10). In addition, 0.5 x 0.5 cm wooden ledges were attached to the edges of the open arms to prevent falling. It was made of black Plexiglas and set 50 cm above the floor. The 5.1 lux illumination at the central square was used of the maze during all testing. The rats were gently placed on the central square facing to the closed arm individually which allowed freely for 5 minutes for exploration on the maze. The behaviors were recorded by a video tracking system. The numbers of entries, the total time spent in each arm and center square were recorded. Total exploration was calculated by the summation of open and closed arms entries. An entry was considered only when all four paws of the rat were entered in an individual arm. Anxiety index were measured by following equation-
Anxiety index= 1- [(Time spent in open arms/Total time on the maze) + (Number of entries to the open arms/ total exploration on the maze)]/2 [24].
Open-field test (OFT)
Depression-like behaviors in rodents also measured by OFT by evaluating time spending in center zone, number of central zone crossing, locomotor activity and freezing behavior [23, 25, 26]. The open field instruments made of a rectangular area of 76 × 76 cm fenced by a wall (46 cm high) and lux intensity was kept at 130-140 lux. It contained of two zones, peripheral zone and central (34 × 34 cm) which were marked by a white line on the maze. All rats were placed on one specific selected corner of the OFT for 5 min exploration in and its activity was assessed by using SMART program (PanLab, Barcelona, Spain).
Measurement of biochemical profile
Serum levels of free fatty acid (FFA), low density lipoprotein (LDL), high-density lipoprotein (HDL), very low density lipoprotein cholesterol (VLDL), low density lipoprotein (LDL), total cholesterol (TC), triglyceride (TG), leptin and adiponectin and insulin resistance were measured as described [1]. The concentration of IL-6 and TNF-α in brain tissue extracts were quantified with ELISA kits from R&D Systems (Biocalvin Company, Suzhou, China). The serum corticosterone was measured by commercial corticosterone ELISA Kit (Enzo Life Sciences, NY, USA).
Determination of ATP concentration and cellular gene expression
ATP concentration was determined as described previously [27]. Briefly, fresh hippocampal tissue was homogenized in a tissue protein extraction reagents contained solution (Pierce, IL, USA), and ATP was determined in duplicate 50-µl aliquots of the supernatant by using a luciferase bioluminescence assay following the manufacturer’s protocol (ATP Bioluminescence Assay kit CLS II, Roche Applied Science). Light emitted from a luciferase-mediated reaction and determined by a tube luminometer (Berthold Detection Systems GmbH, Pforzheim, Germany) was used to calculate the measured values. Light emission at 10-s interval from a luciferase-mediated reaction was determined in a Lmax microplate luminometer (Molecular Devices, Sunnyvale, CA) to calculate the measured values. The gene expression of NRF1, mtTFA, PGC-1 α, brain derived neurotrophic factor (BDNF) were investigated in hippocampal cell lysates by q RT-PCR as described previously [1]. Primer sequences are shown in Table 1.
Table 1. Primer sequences.
Statistical analysis
Statistical analysis was performed by using Prism 5.03 software (GraphPad Software Inc., San Diego, CA, USA). Data were displayed as mean ± standard error of the mean. The paired Student’s t-test or Bonferroni post hoc test following one-way ANOVA were used. Statistical significance was considered at p < 0.05.
RESULTS
Effect of exercise and oral melatonin on anxiety or depression like behavior
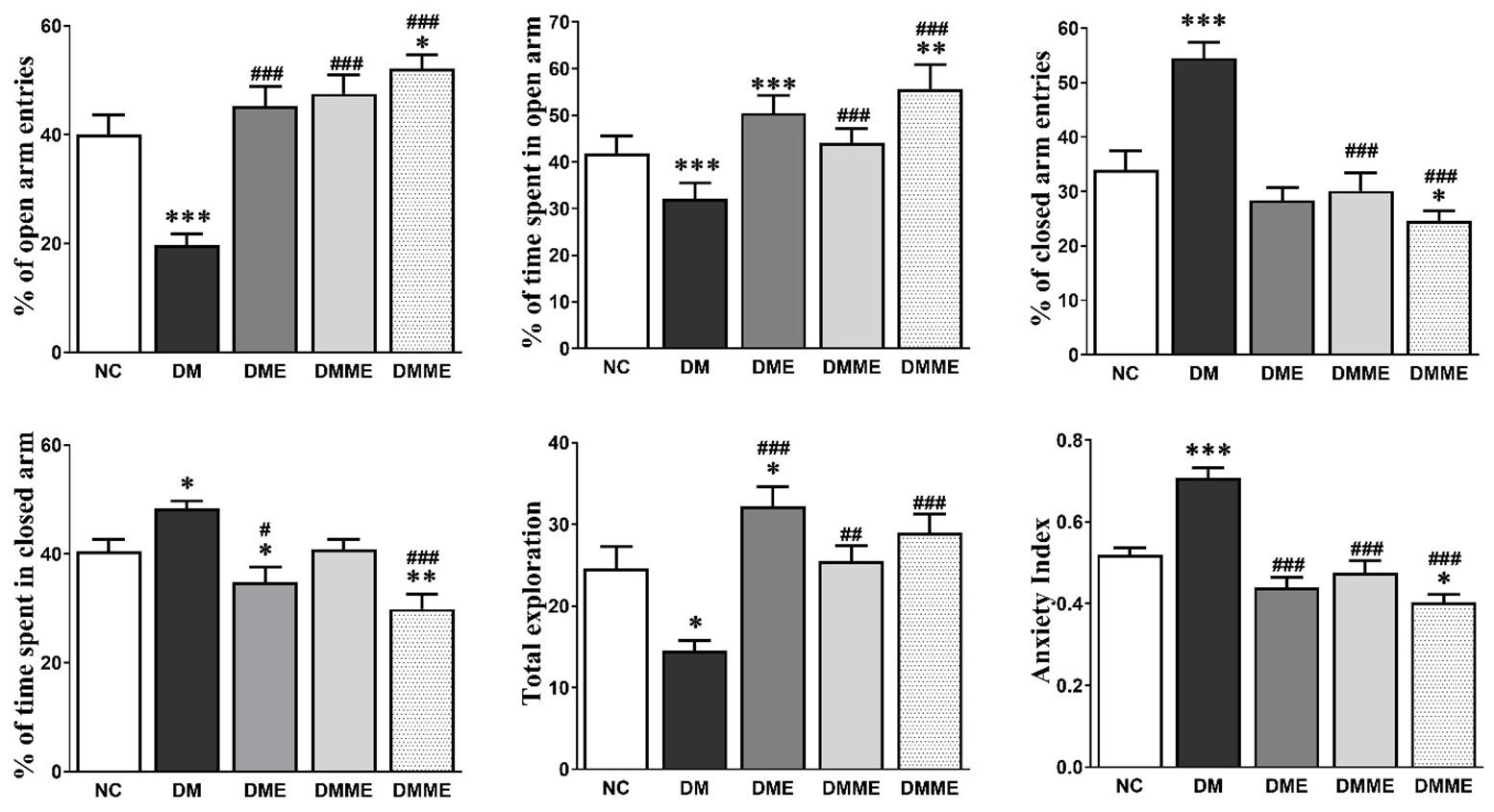
The rats in DM group displayed a significant trend for decrease time spent in the open arms entries (4±1), time spent in open arms (96±9 s) and total exploration (15±1), the NC group (Figure 1), which was reflected to the anxiety index. The anxiety index was NC, DM, DME, DMM and DMME were 0.52±0.02, 0.71±0.02, 0.44±0.02, 0.47±0.03 and 0.40±0.02, respectively.
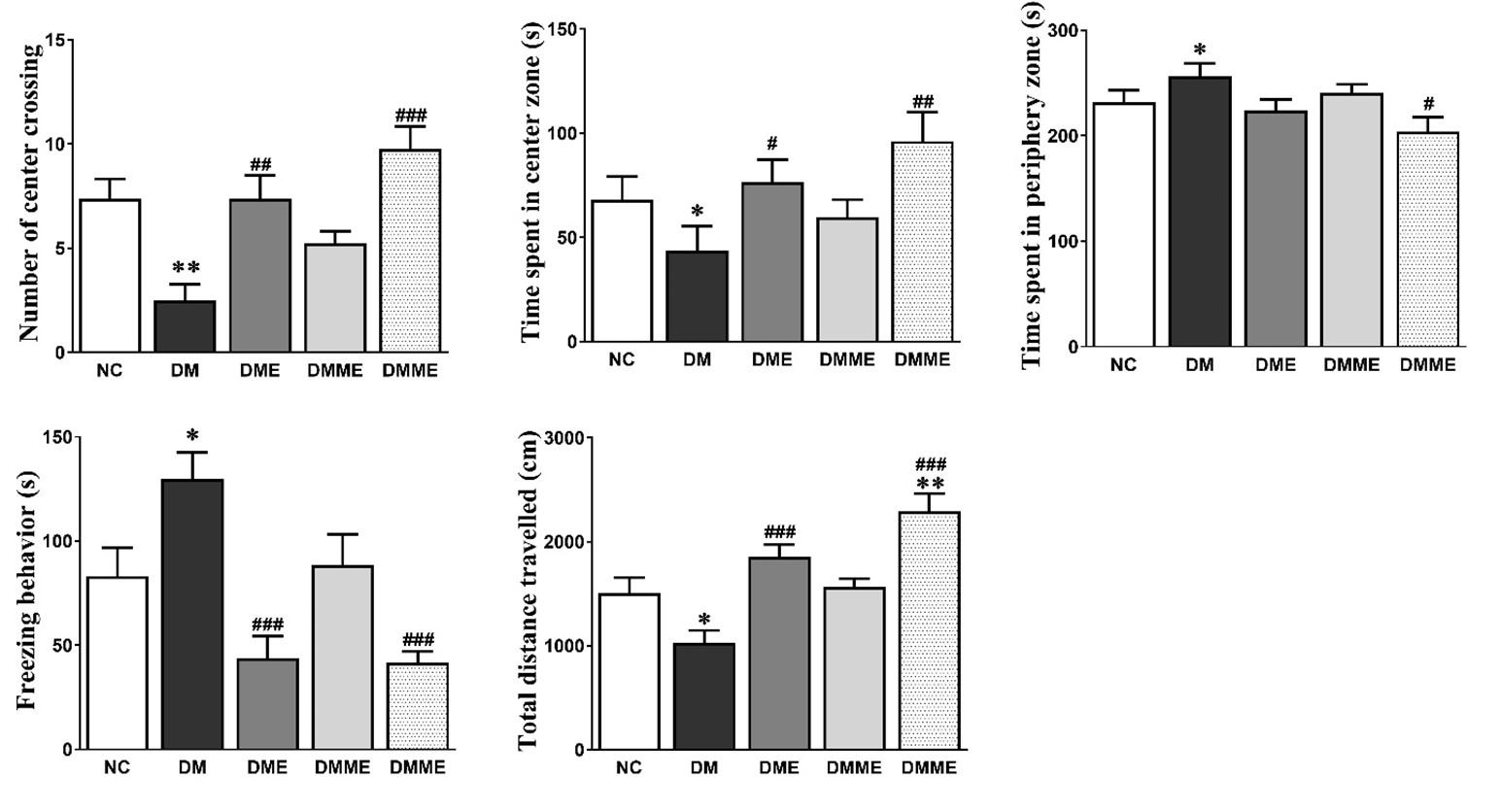
As shown in Figure 2, the reduced total distance travelled (NC, DM, DME, DMM and DMME were, 1505±162, 1030±126, 1858±125, 1569±83 and 2292±185, respectively) number of center crossing (7±2, 3±1, 7±1, 5±1 and 10±2, respectively), and time spent in the central squares of the open field (23, 15, 25, 20 and 32%, respectively) in the DM group were improved significantly by exercise and oral melatonin in DMME group. Moreover, time spent in periphery (77, 86, 75, 80 and 68 % respectively) and freezing behavior was also markedly reduced in DMME group (28, 43, 15, 29 and 14% respectively).
Effects of exercise and oral melatonin on blood and serum biochemistry
The rats of DM group displayed hyperglycemia, hyperinsulinemia, dyslipidemia, hyperleptinemia and hypoadiponectenemia which were gradually corrected by the exercise and oral melatonin. At the end of the experiment, the blood glucose levels of NC, DM, DME, DMM and DMME groups were 6.5±0.2, 17.5±0.9, 13.0±0.4, 11.2±0.8 and 8.8±0.6 mmol/L, respectively (Table 2).
At the end of the day the blood FBG concentration in DM group was significantly elevated compared (p<0.001) to NC group. However, in DME, DMM and DMME group the FBG were reduced significantly by 21.61%, 33.18%, and 39.12%, respectively than DM group (Table 2). The concentration of insulin (0.69 fold), IR (3.37 fold) and leptin (0.88 fold) were significantly elevated and adiponectin (-0.40 fold) were significantly lowered in DM group compared to NC group but were significantly attenuated synergistically by exercise and oral melatonin in DMME group (Table 2). The serum concentration of TG, TC and LDL were increased but HDL decreased in the DM group than NC group. However, these alterations were synergistically attenuated significantly in DMME group. Similarly, serum corticosterone level was also synergistically reduced in DMME group than DM group (Table 2).
Table 2. Effect of exercise and oral melatonin on blood glucose, insulin, insulin resistance, lipid profiles, leptin and adiponectin levels in rats.
Effects of exercise and oral melatonin on hippocampal biochemistry
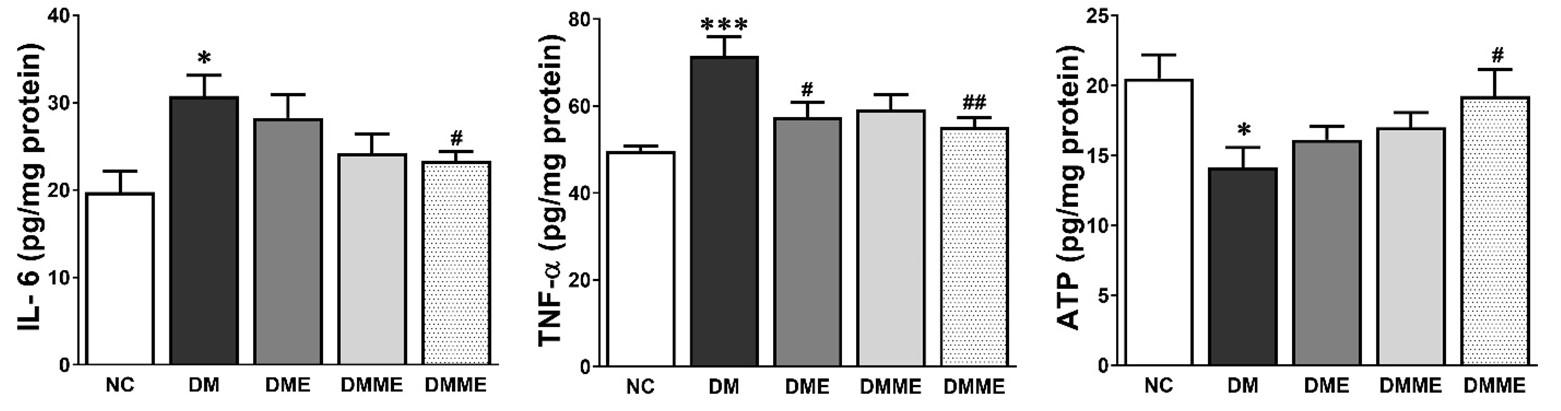
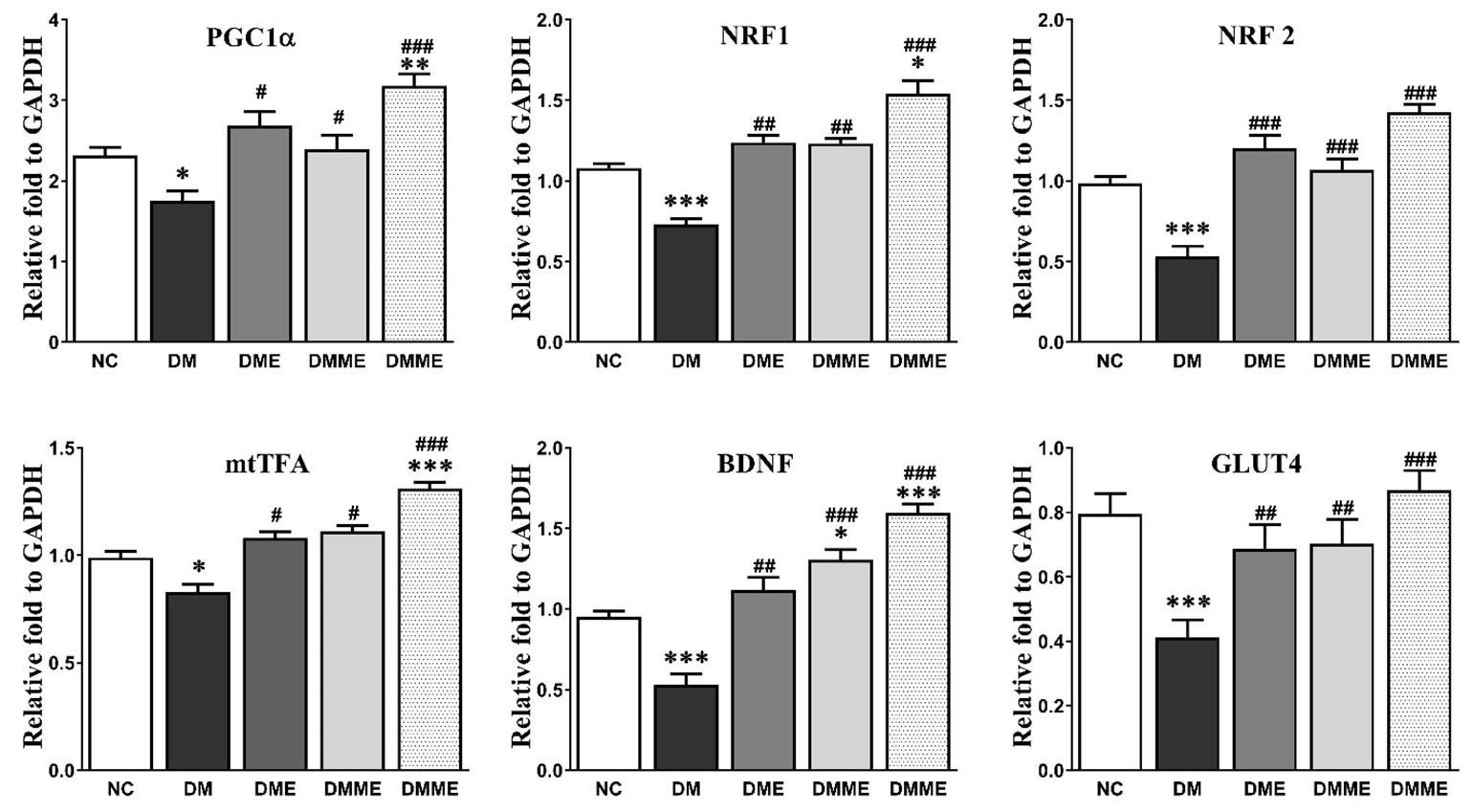
The hippocampal inflammatory cytokines TNF-α and IL-6 were markedly increased in DM group and were reduced by the exercise and oral melatonin in DMME group. The level of ATP was significantly decreased in DM group than NC group which was improved in DMME group. However, these effects were elevated in the treatment groups especially by exercise and oral melatonin (Figure 3). The expression of PGC1α, GLUT4, NRF1, NRF2, mtTFA and BDNF significantly lowered in hippocampus in DM group as compared with NC group. However, the changes of these proteins were effectively up-regulated by exercise and oral melatonin showed in DMME group (Figure 4).
DISCUSSION
The findings indicate that melatonin and exercise combination synergistically ameliorated the anxiety-like behavior in DME rats as represented by increased entries and time spent in center in open field test, reduced open arm entries and time spent in elevated plus maze. Furthermore, combined exercise and oral melatonin synergistically reduced serum corticosterone level, suppressed hippocampus tissue level of inflammation, and improved ATP level and up-regulated the expression of BDNF, GLUT4, PGC-1 α, NRF1, NRF2 and mTFA in hippocampus tissue.
Metabolic syndrome is characterized by a cluster of signs which is strongly associated with T2DM. The predominant indications of this disorder are obesity, hyperinsulinemia, hyperlipidemia, IR and abnormal fasting glucose concentration. When three or more of these signs are present then it is clinically considered as T2DM. These conditions are directly or indirectly associated with depression or neurologic disorders [5-7, 23, 25, 28]. Low level of HDL cholesterol [28, 29] and hypertriglyceridemia [30] independent associations with depression. Although both high and low concentration of serum LDL are correlated with depression, the former one is related to metabolic syndrome [31]. In this study the metabolic syndrome significantly corrected by exercise and oral melatonin evidenced by synergistic reduction of hyperglycemia, IR, hypertriglyceridemia, high level of LDL as well as elevation of lowered HDL level thereby improving anxiety-like behavior in rats. Furthermore, adiponectin and leptin play an important role in the pathophysiology of depressive disorder [32]. Hyperleptinemia is frequently present in obesity and T2DM, is associated to insulin resistance, metabolic syndrome, inflammatory responses and oxidative stress [1, 33, 34] which may lead to depression [35]. Furthermore, hypoadiponectinemia has been also reported to aggravate obesity-related diseases such as T2DM as it decreased fatty acid oxidation, decreased glucose uptake, and increased gluconeogenesis consequently, cause insulin resistance, hyperglycemia, inflammatory response and oxidative stress [36, 37] finally contribute to induce depression [38]. So, controlling hyperleptinemia and hypoadiponectimia is important for ameliorating T2DM and depression. In this study we found that leptin also elevated and adiponectin lowered in the DM group and these were corrected in the DMME group.
Systemic and neuroinflammation plays a critical role to induce depression in diabetes [5, 25, 39]. Inflammatory response suppresses hippocampal neurogenesis and grounds hypothalamic–pituitary–adrenal (HPA) axis hyperactivity increased corticosterone concentration, which is primarily considered to be a result of cytokine induced disturbance of negative feedback via glucocorticoid receptors in the anterior pituitary and hypothalamus [40]. In this experiment, elevated level of IL-6, TNF-α and corticosterone in DM group designates the association of proinflammatory mediators and corticosterone in depression in diabetes. Exercise and oral melatonin combination synergistically reduced the concentration of these pro-inflammatory mediators and corticosterone exhibiting another anti-depressant mechanism. Elevated corticosterone decreases BDNF in hippocampus, consequently increasing depression [41]. Furthermore, BDNF is one of the major neurotrophic factors in the central nervous system which suppress depression [41]. It is regulates neurogenesis, the manipulation of synaptic plasticity, and the release of neurotransmitters [42]. Antidepressants increase hippocampal neurogenesis through mediation of BDNF and its receptor [43]. To explore the neurotrophic mechanism in the antianxiety-like effects of melatonin and exercise combination, BDNF expression in the hippocampus was measured. The results herein indicated that T2DM reduced BDNF expression in the hippocampus which correlates with anxiety-like behavior [43, 44]. Interestingly, melatonin and exercise combination synergistically up-regulated BDNF expression in DMME group, suggesting the potential role of BDNF involvement in the antidepressant-like effects.
The primary fuel of brain for energy metabolism, neural activation and normal function is glucose. Glucose is either oxidized to produce ATP or used to synthesize glycogen [45]. GLUT4 plays a pivotal role in glucose uptake, utilization and the generation of energy in brain tissue [46, 47]. Therefore, lack expression or impairment of GLUT4, deficits in glucose utilization and energy metabolism which is a common feature of T2DM [1, 48, 49]. In this study we found that GLUT4 expression as well as tissue ATP reduced in the hippocampal tissues in diabetes rat which were needed for brain function might be responsible for showing anxiety-like behavior in the diabetes rats in this study. Interestingly, the GLUT4 expression were upregulated, ATP level elevated along with ameliorated anxiety-like behavior by the synergistic action of exercise and oral melatonin in DMME group. Dysfunction of mitochondrial is an important influencing factor in a many disorder such as diabetes, cardiovascular and neurodegenerative (Parkinson’s, Alzheimer’s, and Huntington’s) diseases [1, 50, 51]. Melatonin [15, 16, 52] and exercise [11, 12] are effective tools for up-regulation of PGC1α and to increase mitochondrial biogenesis. Wrann CD et al [53] reported that exercise increased PGC-1a and BDNF expression in the brain. In our previous study we found that exercise and oral melatonin synergistically upregulated PGC1α along with mtFA, NRFs, and GLUT4 expression in muscle and cardiac tissue thereby ameliorating glucose metabolism and diabetes induced cardiac dysfunction. Here, we investigated effects on hippocampal tissue and found that melatonin and exercise synergistically upregulated PGC1α, improved mitochondrial biogenesis in hippocampal tissue manifested by upregulation of NRFs, mtTFA and GLUT4 expression. Importantly, these protein up-regulation and mitochondrial biogenesis play a vital role in the metabolism of glucose [1] and may contribute to ameliorate brain function [45, 48, 54] and thereby attenuating anxiety-like behavior. Moreover, PGC1α primarily regulates mitochondrial biogenesis which regulates oxidative metabolism, systemic inflammation, increases energy expenditure and manipulates glucose homeostasis [55]. Thus, improvement of metabolic syndrome and neuro-behavioral function in DMME could be associated with the upregulation of GLUT4, PGC1α and boosting up mitochondrial biogenesis [1, 49, 56].
CONCLUSIONS
A combinatorial therapy of melatonin and exercise attenuated metabolic syndrome and normalized anxiety and depressive mood in type-2 diabetic rats by regulating IR, hyperlipidemia, leptin, adiponectin, inflammatory cytokines and corticosterone level and up-regulation of GLUT4, PGC-1α, mitochondrial biogenesis and ATP level in hippocampus. Thus, exercise and oral melatonin may rebound in popularity as a treatment tool in T2DM patient with metabolic, anxiety and depression syndrome.
ACKNOWLEDGEMENT
This research was supported by a research fund of the R&D project of KNOTUS Co., Ltd.
AUTHOR CONTRIBUTIONS
MMR and SK designed the experiment and draft the manuscript Conceptualization, MMR, and SK. Methodology and data collection: MMR, HYJ, and SJP; Data curation and analysis: MMR, and SK. Writing—original draft preparation: MMR. Funding acquisition: SK. All authors have revised and agreed to the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Rahman MM, Kwon HS, Kim MJ, Go HK, Oak MH, Kim DH. Melatonin supplementation plus exercise behavior ameliorate insulin resistance, hypertension and fatigue in a rat model of type 2 diabetes mellitus. Biomedicine & pharmacotherapy 2017;92:606-14.
- [2]Rahman MM, Kim MJ, Kim JH, Kim SH, Go HK, Kweon MH, et al. Desalted Salicornia europaea powder and its active constituent, trans-ferulic acid, exert anti-obesity effects by suppressing adipogenic-related factors. Pharmaceutical biology 2018;56:183-91.
- [3]El-Marasy SA, Abdallah HM, El-Shenawy SM, El-Khatib AS, El-Shabrawy OA, Kenawy SA. Anti-depressant effect of hesperidin in diabetic rats. Canadian journal of physiology and pharmacology 2014;92:945-52.
- [4]Go HK, Rahman MM, Kim GB, Na CS, Song CH, Kim JS, et al. Antidiabetic Effects of Yam (Dioscorea batatas) and Its Active Constituent, Allantoin, in a Rat Model of Streptozotocin-Induced Diabetes. Nutrients 2015;7:8532-44.
- [5]Stuart MJ, Baune BT. Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neuroscience and biobehavioral reviews 2012;36:658-76.
- [6]Naicker K, Johnson JA, Skogen JC, Manuel D, Overland S, Sivertsen B, et al. Type 2 Diabetes and Comorbid Symptoms of Depression and Anxiety: Longitudinal Associations With Mortality Risk. Diabetes care 2017;40:352-8.
- [7]Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs 2015;75:577-87.
- [8]Tiller JW. Depression and anxiety. The Medical journal of Australia 2013;199:S28-31.
- [9]Hofmeijer-Sevink MK, Batelaan NM, van Megen HJ, Penninx BW, Cath DC, van den Hout MA, et al. Clinical relevance of comorbidity in anxiety disorders: a report from the Netherlands Study of Depression and Anxiety (NESDA). Journal of affective disorders 2012;137:106-12.
- [10]Knight JA. Physical inactivity: associated diseases and disorders. Annals of clinical and laboratory science 2012;42:320-37.
- [11]Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. The Journal of biological chemistry 2011;286:10605-17.
- [12]Miotto PM, Holloway GP. Exercise-induced reductions in mitochondrial ADP sensitivity contribute to the induction of gene expression and mitochondrial biogenesis through enhanced mitochondrial H2O2 emission. Mitochondrion 2018.
- [13]Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dynamic medicine : DM 2009;8:1.
- [14]Rahman MM, Lee SJ, Mun AR, Adam GO, Park RM, Kim GB, et al. Relationships between blood Mg2+ and energy metabolites/enzymes after acute exhaustive swimming exercise in rats. Biological trace element research 2014;161:85-90.
- [15]Yu L, Gong B, Duan W, Fan C, Zhang J, Li Z, et al. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1alpha-SIRT3 signaling. Scientific reports 2017;7:41337.
- [16]Cardinali DP, Pagano ES, Scacchi Bernasconi PA, Reynoso R, Scacchi P. Melatonin and mitochondrial dysfunction in the central nervous system. Hormones and behavior 2013;63:322-30.
- [17]Zephy D, Ahmad J. Type 2 diabetes mellitus: Role of melatonin and oxidative stress. Diabetes & metabolic syndrome 2015;9:127-31.
- [18]Peschke E. Melatonin, endocrine pancreas and diabetes. Journal of pineal research 2008;44:26-40.
- [19]Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Medicine and science in sports and exercise 2010;42:2282-303.
- [20]Mei Q, Diao L, Xu JM, Liu XC, Jin J. A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta pharmacologica Sinica 2011;32:495-502.
- [21]Kantar S, Turkozkan N, Bircan FS, Pasaoglu OT. Beneficial effects of melatonin on serum nitric oxide, homocysteine, and ADMA levels in fructose-fed rats. Pharmaceutical biology 2015;53:1035-41.
- [22]Chen CW, Cheng HH. A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. The Journal of nutrition 2006;136:1472-6.
- [23]Rebolledo-Solleiro D, Roldan-Roldan G, Diaz D, Velasco M, Larque C, Rico-Rosillo G, et al. Increased anxiety-like behavior is associated with the metabolic syndrome in non-stressed rats. PloS one 2017;12:e0176554.
- [24]Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2012;37:350-63.
- [25]Aswar U, Chepurwar S, Shintre S, Aswar M. Telmisartan attenuates diabetes induced depression in rats. Pharmacological reports : PR 2017;69:358-64.
- [26]Qiu ZK, He JL, Liu X, Zhang GH, Zeng J, Nie H, et al. The antidepressant-like activity of AC-5216, a ligand for 18KDa translocator protein (TSPO), in an animal model of diabetes mellitus. Scientific reports 2016;6:37345.
- [27]Chuang YC, Lin JW, Chen SD, Lin TK, Liou CW, Lu CH, et al. Preservation of mitochondrial integrity and energy metabolism during experimental status epilepticus leads to neuronal apoptotic cell death in the hippocampus of the rat. Seizure 2009;18:420-8.
- [28]Dunbar JA, Reddy P, Davis-Lameloise N, Philpot B, Laatikainen T, Kilkkinen A, et al. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes care 2008;31:2368-73.
- [29]Maes M, Smith R, Christophe A, Vandoolaeghe E, Van Gastel A, Neels H, et al. Lower serum high-density lipoprotein cholesterol (HDL-C) in major depression and in depressed men with serious suicidal attempts: relationship with immune-inflammatory markers. Acta psychiatrica Scandinavica 1997;95:212-21.
- [30]Kamezaki F, Sonoda S, Nakata S, Okazaki M, Tamura M, Abe H, et al. Elevated depressive symptoms are associated with hypertriglyceridemia in Japanese male workers. Internal medicine 2011;50:2485-90.
- [31]Persons JE, Fiedorowicz JG. Depression and serum low-density lipoprotein: A systematic review and meta-analysis. Journal of affective disorders 2016;206:55-67.
- [32]Carvalho AF, Rocha DQ, McIntyre RS, Mesquita LM, Kohler CA, Hyphantis TN, et al. Adipokines as emerging depression biomarkers: a systematic review and meta-analysis. Journal of psychiatric research 2014;59:28-37.
- [33]de Carvalho-Ferreira JP, Masquio DC, da Silveira Campos RM, Dal Molin Netto B, Corgosinho FC, Sanches PL, et al. Is there a role for leptin in the reduction of depression symptoms during weight loss therapy in obese adolescent girls and boys? Peptides 2015;65:20-8.
- [34]Pandey G, Shihabudeen MS, David HP, Thirumurugan E, Thirumurugan K. Association between hyperleptinemia and oxidative stress in obese diabetic subjects. Journal of diabetes and metabolic disorders 2015;14:24.
- [35]Milaneschi Y, Simonsick EM, Vogelzangs N, Strotmeyer ES, Yaffe K, Harris TB, et al. Leptin, abdominal obesity, and onset of depression in older men and women. The Journal of clinical psychiatry 2012;73:1205-11.
- [36]Sheng T, Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. Journal of genetics and genomics = Yi chuan xue bao 2008;35:321-6.
- [37]Caselli C. Role of adiponectin system in insulin resistance. Molecular genetics and metabolism 2014;113:155-60.
- [38]Chan JS, Li A, Ng SM, Ho RT, Xu A, Yao TJ, et al. Adiponectin Potentially Contributes to the Antidepressive Effects of Baduanjin Qigong Exercise in Women With Chronic Fatigue Syndrome-Like Illness. Cell transplantation 2017;26:493-501.
- [39]Benatti C, Blom JM, Rigillo G, Alboni S, Zizzi F, Torta R, et al. Disease-Induced Neuroinflammation and Depression. CNS & neurological disorders drug targets 2016;15:414-33.
- [40]Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nature neuroscience 2008;11:309-17.
- [41]Huang Z, Zhong XM, Li ZY, Feng CR, Pan AJ, Mao QQ. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neuroscience letters 2011;493:145-8.
- [42]Bae JS, Han M, Shin HS, Shon DH, Lee ST, Shin CY, et al. Lycopersicon esculentum Extract Enhances Cognitive Function and Hippocampal Neurogenesis in Aged Mice. Nutrients 2016;8.
- [43]Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neuroscience and biobehavioral reviews 2013;37:1346-62.
- [44]Li FH, Yu HT, Xiao L, Liu YY. Response of BAX, Bcl-2 Proteins, and SIRT1/PGC-1alpha mRNA Expression to 8-Week Treadmill Running in the Aging Rat Skeletal Muscle. Advances in experimental medicine and biology 2016;923:283-9.
- [45]Rao J, Oz G, Seaquist ER. Regulation of cerebral glucose metabolism. Minerva endocrinologica 2006;31:149-58.
- [46]Alam F, Islam MA, Khalil MI, Gan SH. Metabolic Control of Type 2 Diabetes by Targeting the GLUT4 Glucose Transporter: Intervention Approaches. Current pharmaceutical design 2016;22:3034-49.
- [47]de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Current Alzheimer research 2012;9:35-66.
- [48]Matioli M, Nitrini R. Mechanisms linking brain insulin resistance to Alzheimer’s disease. Dementia & neuropsychologia 2015;9:96-102.
- [49]de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Current opinion in investigational drugs 2009;10:1049-60.
- [50]Jesse S, Bayer H, Alupei MC, Zugel M, Mulaw M, Tuorto F, et al. Ribosomal transcription is regulated by PGC-1alpha and disturbed in Huntington’s disease. Scientific reports 2017;7:8513.
- [51]Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. Journal of cell science 2012;125:4963-71.
- [52]Tan DX, Manchester LC, Qin L, Reiter RJ. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. International journal of molecular sciences 2016;17.
- [53]Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell metabolism 2013;18:649-59.
- [54]Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Progress in neurobiology 2013;108:21-43.
- [55]Correia JC, Ferreira DM, Ruas JL. Intercellular: local and systemic actions of skeletal muscle PGC-1s. Trends in endocrinology and metabolism: TEM 2015;26:305-14.
- [56]Neth BJ, Craft S. Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Frontiers in aging neuroscience 2017;9:345.