Multidisciplinary approaches to coping with neurodegenerative disorders amid COVID-19 pandemic
Abstract
Neurodegenerative disorders, including Alzheimer’s and Parkinson’s, are the leading causes of dementia in the elderly. In the coming days, an alarming upsurge of dementia patients is expected with increasing life expectancy. This is the scenario not only in the developed world but also in the developing world, where older people live in vulnerable situations. Even in the COVID-19 (coronavirus disease-19) pandemic, the situation has worsened. Due to the limitations of conventional therapeutic strategies, it is necessary to explore integrated approaches consisting of both pharmacological and non-pharmaceutical interventions. As existing anti-dementia drugs pose many adverse effects on patients, pharmacological intervention through naturally occurring agents should be employed to explore targeted therapy. Alongside, non-pharmacological interventions such as cognitive and motor rehabilitation, occupational therapy, and psychological therapy need to be explored. From this perspective, multidisciplinary approaches need to be employed in order to develop a sustainable patient-friendly treatment strategy for the management of these emerging health issues with tremendous social burdens.
Keywords
INTRODUCTION
Neurological disorders, particularly those associated with dementia such as Alzheimer’s disease and Parkinson’s disease, constitute a major public health issue in elderly people. Globally, there are approximately 50 million people over the age of 65 with dementia, and 70% of them have Alzheimer’s disease (AD), and Parkinson’s disease stands next [1]. Aging is one of the most prominent risk factors for dementia that increases the incidence of neurological disorders [2, 3]. As life expectancy increases with the improved lifestyle, the incidence of dementia is expected to be increased in the future. More than any other natural calamities, the COVID-19 pandemic, in particular, puts elderly people with dementia at greater risk. Because of their multifactorial nature and the underlying complications such as COVID-19, the management of neurodegenerative disorders calls for an integrated approach.
Despite tremendous efforts from the scientific community, no successful therapeutic agent is available that can interfere or reverse disease pathology [4]. The only therapy that is currently in use is symptomatic and does not play any interfering role in disease progression, rather poses several adverse effects on patients. In order to accelerate the development of clinical agents, there is an urgent need to pay for collaborative efforts from multidisciplinary fields. Along with the pharmacological approach, strategies that include non-pharmacological approaches need to be incorporated to better cope with the growing incidence of these diseases.
This review sheds light on the pathobiology, and the prevalence and current treatment strategy of neurodegenerative disorders. Advances in pharmacological intervention, particularly those of natural origin, with a special focus on phytochemicals are outlined. An integrated approach combining pharmacological and nonpharmacological interventions to curbing the incidence of neurodegenerative disorders has been discussed.
METHODOLOGY
Online databases, namely PubMed, Web of Science, Scopus, and Google Scholar were accessed to retrieve the information using a pair combination of keywords such as neurological disorders, dementia, Alzheimer’s and Parkinson’s, pathobiology, epidemiology, multidisciplinary approach, pharmacological interventions, non-pharmaceutical interventions, and COVID-19.
HALLMARKS OF NEURODEGENERATIVE DISORDERS
Protein homeostasis (proteostasis) is crucial for maintaining the physiological function of the brain [5]. Impaired protein homeostasis and the resulting protein misfolding constitute major factors contributing to the pathobiology of neurological disorders [2]. It has been ascertained that the protein homeostasis system usually reduces protein aggregation by either correcting or degrading misfolded protein after the translation through chaperone function or autophagy mechanisms [6]. However, under proteotoxic stress, these homeostasis systems are swamped by the overproduced misfolded proteins; thus, misfolded proteins, which are escaped from these systems, are aggregated into high oligomers and amorphous assemblies, leading to high ordered amyloid fibrils and plaques. This scenario is also supported by many other signaling cascades, including environmental changes, post‐translational modifications, mitochondrial dysfunction, calcium-induced protein misfolding, and post‐translational modifications, affecting protein homeostasis systems and promotes protein misfolding bidirectionally [7]. Furthermore, excessive intracellular calcium accumulation caused NMDA receptor overactivation, leading to ROS/RNS production and endoplasmic reticulum (ER) stress. Oxidative stress, together with misfolded protein, promotes the activation of microglia and astrocytes which release inflammatory mediators and reactive free radicals that take part in neuroinflammation and thereby damage the blood-brain barrier [8]. Neuroinflammation has a dual role in the brain, where mild inflammation provides immunity, while chronic inflammation causes tissue damage. Neuroinflammation generates the “vicious circle” phenomena, triggering an increased level of ROS/RNS, which reduces protein homeostasis capacity, which produces additional misfolded proteins and protein aggregates, directing to mitochondrial dysfunction, and neuronal injury [9, 10].
EPIDEMIOLOGY AND CURRENT MANAGEMENT STRATEGY OF NEURODEGENERATIVE DISORDERS
The two most prevalent neurodegenerative disorders include Alzheimer’s and Parkinson’s diseases that represent primary causes of dementia.
Alzheimer’s disease
Alzheimer’s disease is the most common neurodegenerative disorder worldwide and is responsible for the impairment of numerous key features in cognitive domains. For example, Alzheimer’s disease (AD) among the elderly affects not only mood, spatial abilities, functional execution, but also language and memory processes [11]. Currently, AD prevails in approximately six million Americans aged 65 and over in the United States and is estimated to be 13.8 million by 2050 and is the sixth leading cause of death [1]. Pathological confirmation of AD is primarily characterized by the extracellular formation of amyloid plaques due to the aggregation of amyloid-beta (Aβ) and the intraneuronal deposition of neurofibrillary tangles (NFT) by hyperphosphorylated tau protein in the brain. With the multifunctional etiology and complexity of the disease, nearly all the drug treatments tested for AD have failed so far to show any efficacy or most of them are symptomatic. However, clearance of Aβ and phosphorylated tau depositions could be a curative for AD treatment. Indeed, several potential pathways have been shown to be involved in Aβ clearance and could be a curative for improving brain functions [12]. In addition, tau-targeting therapies have shown promise in numerous preclinical studies to improve cognitive impairments in AD [13]. It is also known that cholinergic synaptic transmission is known to be involved in memory processing in the main brain region, the hippocampus, and alterations with this neurotransmitter result in the degeneration of cholinergic neurons. Similarly, evidence from the post-mortem AD brain also shows the declination of choline acetyltransferase (ChAT) enzymes, neurotransmitter choline acetyltransferase (Ach) and its nicotinic and muscarinic receptors in the cerebral cortex and hippocampus [14]. Regarding the treatment of AD, most of the drugs currently available on the market are therefore predominantly based on the inhibition of cholinesterase, although their efficacy remains questionable as many of them may possess adverse side effects and are unable to fully comply to halt the progression of the disease [15].
Parkinson’s disease
Parkinson’s disease, also known as Lewy Body disease, is a chronic, progressive neurodegenerative condition of aging and is associated with motor dysfunction. Abnormality in motor function such as postural reflex, resting tremor, bradykinesia, and rigidity is a common feature in Parkinson’s disease (PD), however, variable non-motor constellation symptoms that are autonomic, sensory, cognitive, and psychiatric changes have also been well documented. PD is the second most common neurodegenerative disorder after AD, affecting more than 6 million people worldwide and is expected to double by 2040 [16]. Due to a lack of knowledge regarding the underlying pathobiology of PD, there are currently no complete curative therapies. Although some of the symptomatic treatments are available, it has very few or no capabilities to halt the disease progression. So even though U.S. FDA-approved levodopa is an effective treatment option especially for early-stage PD that improves the motor features dramatically, however, prolonged use of this drug results in significant adverse side effects [17]. There is therefore novel method such as restoration of striatal dopamine by restoring dopamine-producing cells using stem cell-derived neurons [18] or reduction in α-Synuclein production either halting the translation of the α-synuclein gene [19] or by enhancing its clearance [20] or restoration of the nigrostriatal pathway [21] could be a useful therapy for prevention of ongoing neurodegeneration and progression of the disease.
MANAGEMENT OF NEURODEGENERATIVE DISORDERS THROUGH MULTIDISCIPLINARY APPROACHES
Being multifactorial diseases, the proper management of neurodegenerative disorders requires a multidisciplinary approach that represents pharmacological and non-pharmacological interventions (Figure 1). While these approaches are individually inadequate, their combination may hold substantial clinical prospects against neurodegenerative disorders.
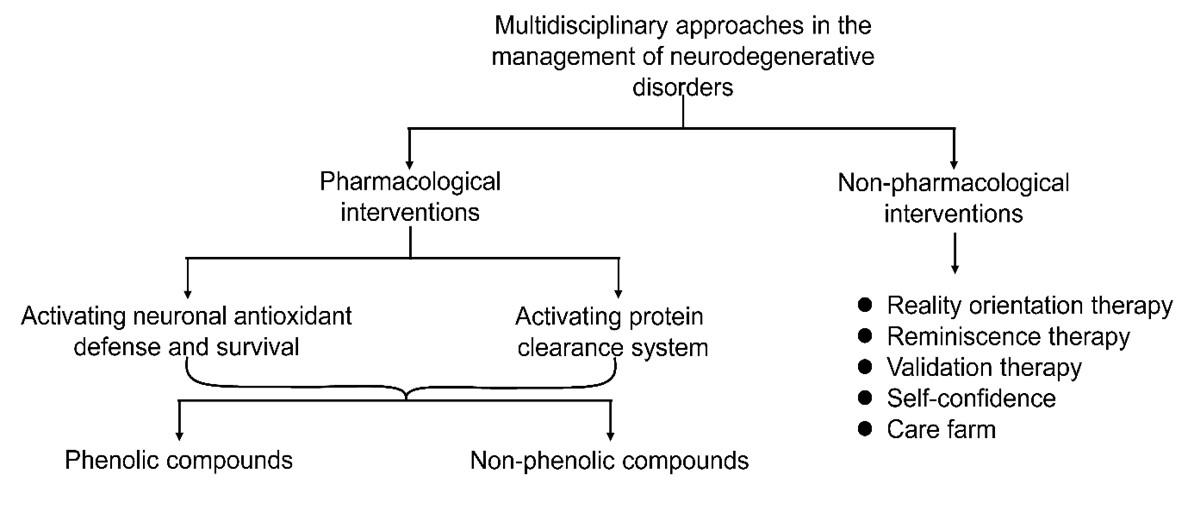
Pharmacological interventions
Several compounds have shown pharmacological potential against pathological outcomes of neurodegenerative disorders. Compounds that confer neuroprotection through activating antioxidant and pro-survival systems, and protein clearance systems are of particular importance as degenerating neurons suffer from the exhausted antioxidant mechanism and compromised cell survival system [22-25] (Figure 2). Of neuroprotective agents, natural products, especially phytochemicals, offer a promising alternative to synthetic chemicals [26-42]. In addition to neuroprotective agents, natural substances that are prospective against COVID-19 may also help patients with COVID-19 and associated complications [43-47].
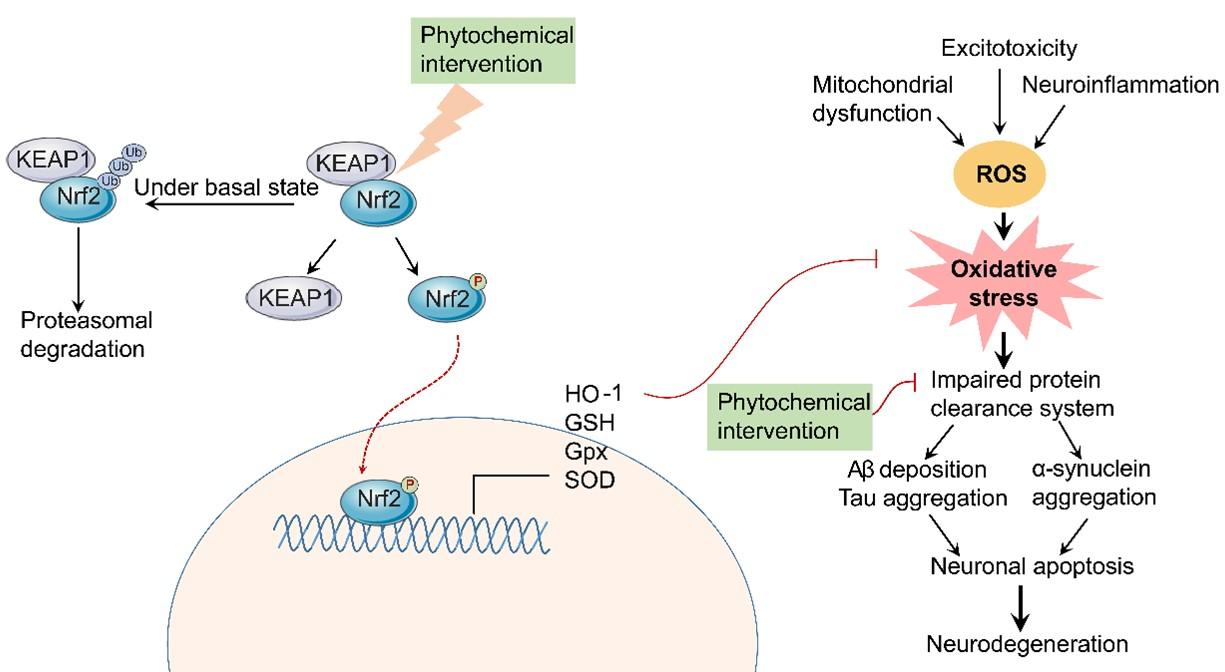
Activating neuronal antioxidant defense and survival by natural compounds
Phenolic compounds
Neuroprotective potentials of numerous non-phenolics natural products are frequently studied against OS in AD and other NDDs models (Table 1). Brassicaphenanthrene A, a non-phenolic compound from Brassica rapa showed HT-22 neuronal cell protection against excitotoxicity evidenced by increased HO-1, Nrf2 (its content and translocation) and, GSH, glutamine cysteine ligase, ARE promoter activity and phosphorylation Akt via regulating JNK and PI3K/Akt regulatory pathways [55]. Similarly, Acerogenin A, extracted from Acer nikoense, mediated activation of the PI3K/Akt/Nrf2/HO-1 pathway in HT22 cells displayed protection against glutamate-mediated oxidative injury [56]. It decreased ROS production and enhanced Nrf2 translocation, HO-1 expression and phosphorylation consequently protecting cell death [56]. Besides, several bio-active non-phenolic compounds have efficient neuroprotective effects and thus, enhance their implications in alleviating neurodegenerative diseases.
Non-phenolic compounds
Neuroprotective potentials of numerous non-phenolics natural products are frequently studied against OS in AD and other NDDs models (Table 1). Brassicaphenanthrene A, a non-phenolic compound from Brassica rapa showed HT-22 neuronal cell protection against excitotoxicity evidenced by increased HO-1, Nrf2 (its content and translocation) and, GSH, glutamine cysteine ligase, ARE promoter activity and phosphorylation Akt via regulating JNK and PI3K/Akt regulatory pathways [55]. Similarly, Acerogenin A, extracted from Acer nikoense, mediated activation of the PI3K/Akt/Nrf2/HO-1 pathway in HT22 cells displayed protection against glutamate-mediated oxidative injury [56]. It decreased ROS production and enhanced Nrf2 translocation, HO-1 expression and phosphorylation consequently protecting cell death [56]. Besides, several bio-active non-phenolic compounds have efficient neuroprotective effects and thus, enhance their implications in alleviating neurodegenerative diseases.
Table 1. Natural compounds activating cell survival system.
Activating protein clearance system by natural compounds
Phenolic compounds
Several phenolic compounds are found to be effective in clearing protein aggregates (Table 2). For instance, a natural phenolic component of extra-virgin olive oil, oleocanthal, can ameliorate Alzheimer’s disease by enhancing Aβ clearance from the brain through up-regulation of P-glycoprotein (P-gp) and LDL lipoprotein receptor-related protein-1 (LRP1), major Aβ transport proteins, at the blood-brain barrier (BBB) [57]. Oleuropein aglycone activated neuronal autophagy in Huntington disease mice [58]. Another phenolic compound kaempferol, derived from kale, beans, tea, spinach and broccoli also acts as an autophagic enhancer by increasing the microtubule-associated protein light chain-3 (LC3-II) suggesting more general protection in Parkinson’s disease [59].
Table 2. Natural compounds enhancing the clearance of protein aggregates.
Non-phenolic compounds
Researchers are also interested in the non-phenolics natural products to observe their effect on protein aggregates clearance in different neurodegenerative diseases as depicted in Table 2. In a study, transgenic N171-82Q mice model of HD as well as Htt transfected non-neuronal cell model (HEK 293 cells) were monitored for the alleviation of motor dysfunction and cell survival. Diseased mice were treated with 40 mg/ kg BW berberine whereas the HEK 293 cells were treated with 50uM berberine. The compound induced autophagy promoting the degradation of mutant huntingtin reducing the expression of P62 meanwhile elevating the LC3B-II protein expression [60]. The UPS-mediated proteolysis activity of sulforaphane (0.5 mg/kg and 10uM) in GFPu transgenic UPS function model mouse as well as in HeLa and HEK293 cells overexpressed with GFP-tagged Htt-exon1 containing 74Q [61]. Sulforaphane induced both autophagic and proteasomal activities in an in vitro and a transgenic mouse model by reducing mHtt mediated neurotoxicity and accumulation in HD cell models. A significant increment in the caspase-like, trypsin-like and chymotrypsin-like activities including the expression of LC3-I and LC3-II protein levels were reported [61]. Fujikake et al. observed the effect of geldanamycin (500 nM) in Drosophila with Q128 R6/2 HTT N-terminal fragment and mouse model of HTT exon 1 Q150 mutations including the cell lines like COS1 cells expressing N-terminal HTT Q51. Geldanamycin induced multiple molecular chaperones on polyQ-induced neurodegeneration with the increment in Hsp70, Hsp40, and Hsp90 expressions promoting aggregates clearance [62]. Celastrol was treated (1.6 μM) in various cell lines transfected with Q57-YFP to make the HD model in HeLa, PC12 cells, HSF1+/+ as well as HSF1-/- mouse embryo fibroblast (MEF) cells. It significantly up-regulated HSP gene expression (HSF1) promoting the solubility of polyglutamine aggregates in sodium dodecyl sulfate (SDS) with the increased expression of autophagy markers Hsp70 and accelerated the clearance of Q57-YFP aggregates [63]. All these studies provide the significant contribution of non-phenolic natural products in protein aggregates clearance in various neurodegenerative diseases by promoting autophagy or chaperone-mediated pathways.
Nonpharmacological interventions
Non-pharmacological interventions (NPI) represent an important complement to standard pharmacological treatment in various neurodegenerative disorders. Especially in the time of pandemic situation, non-pharmacological strategies instead of therapeutic regimens can be easier to follow by the patients. Accumulating evidence suggests that NPI is not only effective in reducing cognitive decline but also in improving psychosocial problems in those with mild cognitive impairment. Since there is no effective treatment for dementia, what is available is only to relieve symptoms [64], NPI is considered a favorable alternative to preventive strategies, because of no or leas side effects. Furthermore, the efficacy of the currently available drug is very limited and because of brain plasticity, the interest in NPI for managing patients with dementia is expanding day by day.
Patients with dementia have been treated with many non-pharmacological treatments, targeting functional, cognitive, and neuropsychiatric aspects have been proposed [65]. In the cognitive approach, which is also emotion-oriented, patients with dementia can improve cognitive, emotional, and social functioning by some common treatments including, reality orientation therapy, reminiscence therapy, and validation therapy [66]. In addition, self-confidence may also potentially contribute to the management of dementia complicated with pandemic such as COVID-19 [67].
Reality orientation (RO) is a process of cognitive stimulation [68], that helps the patients talk about various arguments related to their daily activities and recent events. Encouraging the patient to be socially connected is an important part of the therapy [69, 70]. Reports suggest that RO therapy presents the patient with continuous memory and orientation information about the personal environment and problems [69, 70].
Another interesting non-pharmacological intervention is “Reminiscence therapy”. This therapy can give a feeling of satisfaction, fulfillment, and comfort, which helps patients with memory and other neurodegenerative disorder. The therapy involves recalling the events from memories. it encourages older patients to communicate and interact with a listener in the present. The therapy settings can be either in a group session or in a one-on-one setting [71]. Digital therapy is also an option, allowing multiple users to participate in therapy at the same time. In addition, Digital RT provides the facility, for example, to upload personal content and to present individual triggers for personal memory [72]. In a recent study, digital therapy has been introduced which can be a solution in the pandemic time for patients.
Physical activity has been suggested as one of the basic methods of improving cognitive function, and it can undoubtedly improve the disease situation of neurodegeneration disorder patients [73]. Regular physical activity helps improve cognition and reduce the risk of AD, dementia, or other NDDs and delay their progression. Several studies reported some promising outcomes with exercise in mild to moderate cognitive impairment in depressed older adults [73]. However, more research is essential in this area to delineate the mechanism of physical activity in NDD as a non-pharmacological intervention.
Psychological therapy is one of the most common and widely known therapy for memory-related diseases. Psychological interventions have been studied very widely in improving the general psychological condition and disease condition of patients [74, 75]. Both one-on-one and group session methods are found effective in dementia and depression-related problems of patients. The overall success of psychiatric interventions in a patient with dementia or mild cognitive impairment depends on the outcomes of depression, anxiety, psychological distress, or mental health-related quality of life. Cognitive-behavioral therapy, psychodynamic therapy, interpersonal therapy, and supportive counseling are the main psychotherapeutic approaches, including evidence of efficacy in treating related disorders in elderly patients.
Apart from these, there are some other therapies or methods that can improve the condition of patients and delay the progression of the disease. Although there is no clear and specific mechanism, the treatment methods with some different kinds of therapy can play a great role in improving a patient’s condition and mental state. Lately, different methods such as art, music, aromatherapy, and meditations have gained attention in the field of non-pharmacological intervention of NDD management. Clinical studies have proved the efficiency of these therapies. Singing and music-with movement have been shown to be effective in people with dementia. In music therapy, singing can create a sense of wellbeing and strengthen the patient’s positive self-esteem, sense of achievement, and sense of kinship [76]. Also in clinical studies, the promising effects of musical interventions such as group music therapy (GMT) and recreational choir singing (RCS) have been shown in elderly dementia patients [77]. Music therapy is generally held by expert personnel, still, it can play a great role in reducing the caregiver burden in a situation when social distancing is mandatory.
Clinical research has also been conducted for proving the efficiency of art-based therapy for patients with memory and neuronal disorders [78, 79]. Art therapy is a treatment for problems of the mind and behavior. Art can be used as a way to express and communicate thoughts and feelings. The goal of art therapy is to help patients in ways that help them change and ‘grow’ on a personal level. Generally, traditional art therapies include mainly simple art activities such as painting with colors, sketching drawing graffiti, making collages and coloring pictures, which guide the patients to express feelings and share stories through the artwork which assist them to vent out emotions, improve attention, release stress and improve mental condition and mood during the treatment process [80, 81]. Creative arts (CA) modalities, including dance movement, drama, visual arts, are also used internationally for the treatment of depression and related symptoms [78]. Meditation is a term that encompasses a wide range of techniques and is an integral part of mindfulness-oriented intervention [82]. In its various forms, meditation has been shown to be associated with a reduction in symptoms in medical and psychiatric conditions, and beneficial brain changes in neuroimaging with long-term practice [83-85]. Practicing mindfulness meditation ultimately develops psychological well-being by increasing mindfulness and weakening responsiveness to mental stimuli by helping to divert attention from stimuli. It has also been found that the practice of meditation regularly is significantly responsible for enhancing cognitive flexibility and attentive effectiveness [86, 87].
In the time of global pandemic like COVID-19, maintaining social distancing and taking safety measurements is a non-negotiable matter for public health. In this situation, managing the health and mental state of patients with a neurodegenerative disorder, especially related to memory impairment is a very critical question. That’s why following some of the non-pharmacological methods for managing the condition can provide lots of benefits. The digital therapies, meetings, and classes will reduce the burden of caregiving to some extent and play important role in patient management.
Care farm
Healing agriculture or care farm is the concept of mentally healing the participants through agricultural activities related to horticulture, animals, and insects, resources, and environment related to gardening, animals, and insects, and ultimately connecting agriculture and people [88, 89]. A large-scale national policy of the dementia national responsibility system and the “Healing Agriculture R&D and Promotion Act”, which were promulgated in March 2020, laid the foundation for the development of healing agriculture in South Korea. The expansion of the domestic healing farm (care farm) industry is expected as the Ministry of Health and Welfare (MOHW) and Rural Development Administration (RDA) systematize cooperation to cure dementia and vitalizing rural areas. Recently, the Ministry of Health and Welfare (MOHW) announced an expansion of the use of healing programs for strengthening the cognitive function of the Dementia Relief Center (DRC) and healing programs of care farm through a cooperative MOU with the Rural Development Administration.
Currently, the domestic care farm industry is in its infancy, but overseas is receiving systematic support from the state by recognizing the effects of improving healing, education, and quality of life based on the pluralistic functions of agriculture [90]. In South Korea, the combination of a relief center and a healing farm will provide priority support to dementia patients. Accordingly, additional expenses such as fees for use of agricultural healing facilities were stipulated to be available in the budget for the relief center. It is said that there is a high possibility that the form of a healing farm will develop into a form to support dementia patients. The key to strengthening dementia treatment and revitalizing agriculture is to establish a smooth collaboration system.
The Promotion Agency decided that the purpose of this collaboration was to develop a source of income for farmers and to promote the emotional stability of the people through the commercialization of agricultural healing functions through plants, animals, and insects. The initial model conceived for this is the field application of a healing tour program. It is divided into exchange healing type (increased life satisfaction), relaxation healing type (improving subjective happiness), and exercise healing type (increasing recovery elasticity and subjective vitality). In addition, it is planning to expand the operation of a dementia program to the village and a healing farming program linked to the safety center and farm.
As the development of healing farms in the area of dementia is predicted, it is expected that a smooth connection with the development of agriculture should be achieved through the development of various programs and verification of the effectiveness. Recently, healing farming has been in the spotlight as one of the most effective programs for dementia prevention and cognitive support. In particular, it is attracting attention because it is an outdoor activity that has a low risk of infection and a sufficient distance when indoor activities are difficult due to COVID19. Therefore, care farm is expected to promote both rural economy and mental health.
CONCLUSIONS AND FUTURE DIRECTIONS
The current review highlights the prevalence of major neurological disorders that underlie dementia in the elderly and discussed multidisciplinary approaches, including pharmacological and nonpharmacological interventions to address these emerging public health issues. Pharmacological intervention through natural agents, particularly phytochemicals, hold significant promise in the development of therapeutics for neurological disorders, while existing drugs pose many side effects. However, phytochemical-based drug development has several limitations, including aqueous instability and poor bioavailability, and thus the need for advanced drug delivery systems such as nanoparticle-mediated drug delivery. On the other hand, nonpharmacological interventions represent a promising tool that can be combined with a pharmacological strategy to better cope with these multifactorial diseases. An integrated approach is, therefore, crucial for developing a sustainable strategy for the management of patients with neurodegenerative disorders that may be complicated by the current COVID-19 pandemic.
AUTHOR CONTRIBUTIONS
MAH planned and drafted the manuscript. RD contributed to manuscript preparation. BT contributed to manuscript and table preparation. YAM contributed to manuscript and table preparation. DFO contributed to manuscript preparation. MNH contributed to manuscript preparation. AAMS contributed to manuscript and table preparation. MDNM planned and revised the manuscript. ISM planned, supervised, and revised the manuscript. All authors read and approved the manuscript.
ACKNOWLEDGEMENT
Our research was supported by the Basic Science Research Program (grant number 2021R1A2C1008564 to ISM) through the National Research Foundation of Korea (NRF) funded by the Korean government Ministry of Science and ICT.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]2020 Alzheimer’s disease facts and figures. Alzheimers Dement 2020;16:391-460. https://doi.org/10.1002/alz.12068
- [2]Dash R, Ali MC, Jahan I, Munni YA, Mitra S, Hannan MA, et al. Emerging potential of cannabidiol in reversing proteinopathies. Ageing Res Rev 2021;65. https://doi.org/10.1016/j.arr.2020.101209
- [3]Dash R, Jahan I, Ali MC, Mitra S, Munni YA, Timalsina B, et al. Potential roles of natural products in the targeting of proteinopathic neurodegenerative diseases. Neurochem Int 2021;145.
- [4]Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. TRCI 2021;7:e12179. https://doi.org/10.1002/trc2.12179
- [5]Hetz C. Adapting the proteostasis capacity to sustain brain healthspan. Cell 2021;184:1545-60. https://doi.org/10.1016/j.cell.2021.02.007
- [6]Gandhi J, Antonelli AC, Afridi A, Vatsia S, Joshi G, Romanov V, et al. Protein misfolding and aggregation in neurodegenerative diseases: a review of pathogeneses, novel detection strategies, and potential therapeutics. Rev Neurosci 2019;30:339-58. https://doi.org/10.1515/revneuro-2016-0035
- [7]Vanni S, Colini Baldeschi A, Zattoni M, Legname G. Brain aging: A Ianus-faced player between health and neurodegeneration. J Neurosci Res 2020;98:299-311. https://doi.org/10.1002/jnr.24379
- [8]Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 2009;27:119-45. https://doi.org/10.1146/annurev.immunol.021908.132528
- [9]Seneci P. Chapter 6 – Targeting Assembly and Disassembly of Protein Aggregates: A Raggle-taggle Bunch with High Hopes. In: Seneci P, editor. Chemical Modulators of Protein Misfolding and Neurodegenerative Disease. San Diego: Academic Press; 2015. p. 173-228.
- [10]Hipp MS, Park SH, Hartl FU. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol 2014;24:506-14. https://doi.org/10.1016/j.tcb.2014.05.003
- [11]Chen X-Q, Mobley WC. Alzheimer Disease Pathogenesis: Insights From Molecular and Cellular Biology Studies of Oligomeric Aβ and Tau Species. Front Neurosci 2019;13:659. https://doi.org/10.3389/fnins.2019.00659
- [12]Sun B-L, Li W-W, Zhu C, Jin W-S, Zeng F, Liu Y-H, et al. Clinical Research on Alzheimer’s Disease: Progress and Perspectives. Neurosci Bull 2018;34:1111-8. https://dx.doi.org/10.1007%2Fs12264-018-0249-z
- [13]Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol 2018;14:399-415. https://doi.org/10.1038/s41582-018-0013-z
- [14]Tata AM, Velluto L, D’Angelo C, Reale M. Cholinergic system dysfunction and neurodegenerative diseases: cause or effect? CNS Neurol Disord Drug Targets 2014;13:1294-303.
- [15]Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol Med Rep 2019;20:1479-87. https://doi.org/10.3892/mmr.2019.10374
- [16]Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459-80. https://doi.org/10.1016/s1474-4422(18)30499-x
- [17]Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev 2013;65:171-222. https://doi.org/10.1124/pr.111.005678
- [18]Barker RA, Parmar M, Studer L, Takahashi J. Human Trials of Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease: Dawn of a New Era. Cell stem cell 2017;21:569-73. https://doi.org/10.1016/j.stem.2017.09.014
- [19]Mittal S, Bjørnevik K, Im DS, Flierl A, Dong X, Locascio JJ, et al. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Sci (New York, NY). 2017;357:891-8. https://doi.org/10.1126/science.aaf3934
- [20]Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, et al. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener 2017;12:11. https://doi.org/10.1186/s13024-017-0154-3
- [21]Kirkeby A, Barker RA. Parkinson disease and growth factors – is GDNF good enough? Nat Rev Neurol 2019;15:312-4. https://doi.org/10.1038/s41582-019-0180-6
- [22]Hannan MA, Dash R, Sohag AAM, Haque MN, Moon IS. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front Mol Neurosci 2020;13. https://doi.org/10.3389/fnmol.2020.00116
- [23]Rahman MA, Rahman MH, Biswas P, Hossain MS, Islam R, Hannan MA, et al. Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer’s disease. Antioxidants 2021;10:1-18. https://doi.org/10.3390/antiox10010023
- [24]Rahman MA, Dash R, Sohag AAM, Alam M, Rhim H, Ha H, et al. Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Mar Drugs 2021;19. https://doi.org/10.3390/md19030167
- [25]Dash R, Mitra S, Ali MC, Oktaviani DF, Hannan MA, Choi SM, et al. Phytosterols: Targeting neuroinflammation in neurodegeneration. Curr Pharm Des 2021;27:383-401. https://doi.org/10.2174/1381612826666200628022812
- [26]Haque MN, Hannan MA, Dash R, Choi SM, Moon IS. The potential LXRβ agonist stigmasterol protects against hypoxia/reoxygenation injury by modulating mitophagy in primary hippocampal neurons. Phytomedicine 2021;81. https://doi.org/10.1016/j.phymed.2020.153415
- [27]Hannan MA, Haque MN, Munni YA, Oktaviani DF, Timalsina B, Dash R, et al. Centella asiatica promotes early differentiation, axodendritic maturation and synaptic formation in primary hippocampal neurons. Neurochem Int 2021;144. https://doi.org/10.1016/j.neuint.2021.104957
- [28]Hannan MA, Sohag AAM, Dash R, Haque MN, Mohibbullah M, Oktaviani DF, et al. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020;69:153201. https://doi.org/10.1016/j.phymed.2020.153201
- [29]Hannan MA, Haque MN, Mohibbullah M, Dash R, Hong YK, Moon IS. Gelidium amansii attenuates hypoxia/reoxygenation-induced oxidative injury in primary hippocampal neurons through suppressing GluN2B expression. Antioxidants 2020;9. https://dx.doi.org/10.3390%2Fantiox9030223
- [30]Hannan MA, Dash R, Haque MN, Mohibbullah M, Sohag AAM, Rahman MA, et al. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar Drugs 2020;18. https://doi.org/10.3390/md18070347
- [31]Hannan MA, Dash R, Haque MN, Choi SM, Moon IS. Integrated system pharmacology and in silico analysis elucidating neuropharmacological actions of withania somnifera in the treatment of alzheimer’s disease. CNS Neurol Disord Drug Targets 2020;19:541-56. https://doi.org/10.2174/1871527319999200730214807
- [32]Hannan MA, Dash R, Mamun Sohag AA, Moon IS. Deciphering molecular mechanism of the neuropharmacological action of fucosterol through integrated system pharmacology and in silico analysis. Mar Drugs 2019;17. https://doi.org/10.3390/md17110639
- [33]Hannan MA, Mohibbullah M, Hong YK, Moon IS. Proteomic Analysis of the Neurotrophic Effect of Gelidium amansii in Primary Cultured Neurons. J Med Food 2017;20:279-87. https://doi.org/10.1089/jmf.2016.3848
- [34]Mohibbullah M, Bhuiyan MMH, Hannan MA, Getachew P, Hong YK, Choi JS, et al. The Edible Red Alga Porphyra yezoensis Promotes Neuronal Survival and Cytoarchitecture in Primary Hippocampal Neurons. Cell Mol Neurobiol 2016;36:669-82. https://doi.org/10.1007/s10571-015-0247-x
- [35]Mohibbullah M, Hannan MA, Choi JY, Bhuiyan MMH, Hong YK, Choi JS, et al. The Edible Marine Alga Gracilariopsis chorda Alleviates Hypoxia/Reoxygenation-Induced Oxidative Stress in Cultured Hippocampal Neurons. J Med Food 2015;18:960-71. https://doi.org/10.1089/jmf.2014.3369
- [36]Bhuiyan MMH, Mohibbullah M, Hannan MA, Hong YK, Choi JS, Choi IS, et al. Undaria pinnatifida promotes spinogenesis and synaptogenesis and potentiates functional presynaptic plasticity in hippocampal neurons. Am J Chin Med 2015;43:529-42. https://doi.org/10.1142/s0192415x15500330
- [37]Bhuiyan MMH, Mohibbullah M, Hannan MA, Hong YK, Han CH, Kim YK, et al. The neuritogenic and synaptogenic effects of the ethanolic extract of radix Puerariae in cultured rat hippocampal neurons. J Ethnopharmacol 2015;173:172-82. https://doi.org/10.1016/j.jep.2015.07.013
- [38]Hannan MA, Mohibbullah M, Hwang SY, Lee K, Kim YC, Hong YK, et al. Differential neuritogenic activities of two edible brown macroalgae, undaria pinnatifida and saccharina japonica. Am J Chin Med 2014;42:1371-84. https://doi.org/10.1142/s0192415x14500864
- [39]Hannan MA, Mohibbullah M, Hong YK, Nam JH, Moon IS. Gelidium amansii promotes dendritic spine morphology and synaptogenesis, and modulates NMDA receptor-mediated postsynaptic current. In Vitro Cell Dev Biol Anim 2014;50:445-52. https://doi.org/10.1007/s11626-013-9721-2
- [40]Hannan MA, Kang JY, Mohibbullah M, Hong YK, Lee H, Choi JS, et al. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J Ethnopharmacol 2014;152:142-50. https://doi.org/10.1016/j.jep.2013.12.036
- [41]Hannan MA, Kang JY, Hong YK, Lee H, Choi JS, Choi IS, et al. The marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytother Res 2013;27:21-9. https://doi.org/10.1002/ptr.4684
- [42]Hannan MA, Kang JY, Hong YK, Lee H, Chowdhury MTH, Choi JS, et al. A brown alga Sargassum fulvellum facilitates neuronal maturation and synaptogenesis. In Vitro Cell Dev Biol Anim 2012;48:535-44. https://doi.org/10.1007/s11626-012-9537-5
- [43]Mahamud ASU, Kabir ME, Sohag AAM, Chen C, Hannan MA, Sikder MH, et al. Food-Derived Bioactive Peptides: A Promising Substitute to Chemosynthetic Drugs Against the Dysregulated Renin-Angiotensin System in COVID-19 Patients. J Biol Act Prod Nat 2021:1-31. https://doi.org/10.1080/22311866.2021.1945494
- [44]Islam MN, Hossain KS, Sarker PP, Ferdous J, Hannan MA, Rahman MM, et al. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytother Res 2021;35:1329-44. https://doi.org/10.1002/ptr.6895
- [45]Hannan MA, Rahman MA, Sohag AAM, Uddin MJ, Dash R, Sikder MH, et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021;13:1784. https://doi.org/10.3390/nu13061784
- [46]Hossain KS, Hossain MG, Moni A, Rahman MM, Rahman UH, Alam M, et al. Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. Heliyon 2020;6. https://doi.org/10.1016/j.heliyon.2020.e05798
- [47]Farjana M, Moni A, Sohag AAM, Hasan A, Hannan MA, Hossain MG, et al. Repositioning vitamin c as a promising option to alleviate complications associated with COVID-19. Infect Chemother 2020;52:461-77. https://doi.org/10.3947/ic.2020.52.4.461
- [48]Kwon SH, Ma SX, Hwang JY, Lee SY, Jang CG. Involvement of the Nrf2/HO-1 signaling pathway in sulfuretin-induced protection against amyloid beta25-35 neurotoxicity. Neurosci 2015;304:14-28. https://doi.org/10.1016/j.neuroscience.2015.07.030
- [49]Hui Y, Chengyong T, Cheng L, Haixia H, Yuanda Z, Weihua Y. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-β(1-42) in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem Res 2018;43:297-305. https://doi.org/10.1007/s11064-017-2421-7
- [50]Kong D, Yan Y, He XY, Yang H, Liang B, Wang J, et al. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. BioMed Res Int 2019;2019:8983752. https://doi.org/10.1155/2019/8983752
- [51]Thabet NM, Moustafa EM. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: role of PI3K/AKT/GSK-3β/NRF-2 signalling pathway. Arch Physiol Biochem 2018;124:185-93. https://doi.org/10.1080/13813455.2017.1374978
- [52]Wang D, Liu L, Zhu X, Wu W, Wang Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell Mol Neurobio 2014;34:1209-21. https://doi.org/10.1007/s10571-014-0098-x
- [53]Yu H, Liu P, Tang H, Jing J, Lv X, Chen L, et al. Oleuropein, a natural extract from plants, offers neuroprotection in focal cerebral ischemia/reperfusion injury in mice. Eur J Pharmacol 2016;775:113-9. https://doi.org/10.1016/j.ejphar.2016.02.027
- [54]Li Y, Tian Q, Li Z, Dang M, Lin Y, Hou X. Activation of Nrf2 signaling by sitagliptin and quercetin combination against β-amyloid induced Alzheimer’s disease in rats. Drug Dev Res 2019;80:837-45. https://doi.org/10.1002/ddr.21567
- [55]Lee H, Ko W, Chowdhury A, Li B, Kim SC, Oh H, et al. Brassicaphenanthrene A from Brassica rapa protects HT22 neuronal cells through the regulation of Nrf2‑mediated heme oxygenase‑1 expression. Mol Med Rep 2020;21:493-500. https://doi.org/10.3892/mmr.2019.10824
- [56]Lee DS, Cha BY, Woo JT, Kim YC, Jang JH. Acerogenin A from Acer nikoense Maxim Prevents Oxidative Stress-Induced Neuronal Cell Death through Nrf2-Mediated Heme Oxygenase-1 Expression in Mouse Hippocampal HT22 Cell Line. Molecules 2015;20:12545-57. https://doi.org/10.3390/molecules200712545
- [57]Abuznait AH, Qosa H, Busnena BA, El Sayed KA, Kaddoumi A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: in vitro and in vivo studies. ACS Chem Neurosci 2013;4:973-82. https://doi.org/10.1021/cn400024q
- [58]Luccarini I, Grossi C, Rigacci S, Coppi E, Pugliese AM, Pantano D, et al. Oleuropein aglycone protects against pyroglutamylated-3 amyloid-ß toxicity: biochemical, epigenetic and functional correlates. Neurobiol Aging 2015;36:648-63. https://doi.org/10.1016/j.neurobiolaging.2014.08.029
- [59]Filomeni G, Graziani I, De Zio D, Dini L, Centonze D, Rotilio G, et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson’s disease. Neurobiol Aging 2012;33:767-85. https://doi.org/10.1016/j.neurobiolaging.2010.05.021
- [60]Jiang W, Wei W, Gaertig MA, Li S, Li X-J. Therapeutic effect of berberine on Huntington’s disease transgenic mouse model. PLoS One 2015;10:e0134142. https://doi.org/10.1371/journal.pone.0134142
- [61]Liu Y, Hettinger CL, Zhang D, Rezvani K, Wang X, Wang H. Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential therapeutic reagent for Huntington’s disease. J Neurochem 2014;129:539-47. https://doi.org/10.1111/jnc.12647
- [62]Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. JBC 2008;283:26188-97. https://doi.org/10.1074/jbc.M710521200
- [63]Zhang YQ, Sarge KD. Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response. J Mol Med 2007;85:1421-8. https://doi.org/10.1007/s00109-007-0251-9
- [64]Dash R, Choi HJ, Moon IS. Mechanistic insights into the deleterious roles of Nasu-Hakola disease associated TREM2 variants. Sci Rep 2020;10:1-14. https://doi.org/10.1038/s41598-020-60561-x
- [65]Acevedo A, Loewenstein DA. Nonpharmacological cognitive interventions in aging and dementia. J Geriatr Psychiatry Neurol 2007;20:239-49. https://doi.org/10.1177/0891988707308808
- [66]Finnema E, Dröes RM, Ribbe M, Van Tilburg W. The effects of emotion-oriented approaches in the care for persons suffering from dementia: a review of the literature. Int J Geriatr 2000;15:141-61. https://doi.org/10.1002/(sici)1099-1166(200002)15:2%3C141::aid-gps92%3E3.0.co;2-5
- [67]Hannan MA, Islam MN, Uddin MJ. Self-confidence as an immune-modifying psychotherapeutic intervention for covid-19 patients and understanding of its connection to cns-endocrine-immune axis. JABET 2020;3:14-7. https://doi.org/10.5455/jabet.2020.d151
- [68]Choi J, Twamley EW. Cognitive rehabilitation therapies for Alzheimer’s disease: a review of methods to improve treatment engagement and self-efficacy. Neuropsychol Rev 2013;23:48-62. https://doi.org/10.1007/s11065-013-9227-4
- [69]Camargo CH, Justus FF, Retzlaff G. The effectiveness of reality orientation in the treatment of Alzheimer’s disease. AJADD 2015;30:527-32. https://doi.org/10.1177/1533317514568004
- [70]Spector A, Orrell M, Woods B. Cognitive Stimulation Therapy (CST): effects on different areas of cognitive function for people with dementia. Int J Geriatr 2010;25:1253-8. https://doi.org/10.1002/gps.2464
- [71]Klever S. Reminiscence therapy: Finding meaning in memories. Nursing 2020. 2013;43. https://doi.org/10.1097/01.nurse.0000427988.23941.51
- [72]Moon S, Park K. The effect of digital reminiscence therapy on people with dementia: a pilot randomized controlled trial. BMC Geriatr 2020;20:166. https://doi.org/10.1186/s12877-020-01563-2
- [73]Williams CL, Tappen RM. Exercise training for depressed older adults with Alzheimer’s disease. Aging Ment Health 2008;12:72-80. https://dx.doi.org/10.1080%2F13607860701529932
- [74]Farrand P, Matthews J, Dickens C, Anderson M, Woodford J. Psychological interventions to improve psychological well-being in people with dementia or mild cognitive impairment: systematic review and meta-analysis protocol. BMJ Open 2016;6:e009713. http://dx.doi.org/10.1136/bmjopen-2015-009713
- [75]Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord 2007;101:75-89. https://doi.org/10.1016/j.jad.2006.10.025
- [76]Ray KD, Götell E. The Use of Music and Music Therapy in Ameliorating Depression Symptoms and Improving Well-Being in Nursing Home Residents With Dementia. Front Med (Lausanne) 2018;5:287. https://dx.doi.org/10.3389%2Ffmed.2018.00287
- [77]Gold C, Eickholt J, Assmus J, Stige B, Wake JD, Baker FA, et al. Music Interventions for Dementia and Depression in ELderly care (MIDDEL): protocol and statistical analysis plan for a multinational cluster-randomised trial. BMJ open 2019;9:e023436-e. https://doi.org/10.1136/bmjopen-2018-023436
- [78]Deshmukh SR, Holmes J, Cardno A. Art therapy for people with dementia. CDSR 2018;9:CD011073-CD. https://doi.org/10.1002/14651858.cd011073.pub2
- [79]Uttley L, Stevenson M, Scope A, Rawdin A, Sutton A. The clinical and cost effectiveness of group art therapy for people with non-psychotic mental health disorders: a systematic review and cost-effectiveness analysis. BMC Psychiatry 2015;15:151. https://doi.org/10.1186/s12888-015-0528-4
- [80]Chancellor B, Duncan A, Chatterjee A. Art Therapy for Alzheimer’s Disease and Other Dementias. J Alzheimer’s Dis 2014;39:1-11. https://doi.org/10.3233/jad-131295
- [81]Wang Q-Y, Li D-M. Advances in art therapy for patients with dementia. Chin Nurs Res 2016;3:105-8. https://doi.org/10.1016/j.cnre.2016.06.011
- [82]Keng S-L, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clin Psychol Rev 2011;31:1041-56. https://dx.doi.org/10.1016%2Fj.cpr.2011.04.006
- [83]Wahbeh H, Elsas S-M, Oken BS. Mind–body interventions: Applications in neurology. Neurology 2008;70:2321-8. https://doi.org/10.1212/01.wnl.0000314667.16386.5e
- [84]Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol Med 2010;40:1239-52. https://doi.org/10.1017/s0033291709991747
- [85]Vestergaard-Poulsen P, van Beek M, Skewes J, Bjarkam CR, Stubberup M, Bertelsen J, et al. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2009;20:170-4. https://doi.org/10.1097/wnr.0b013e328320012a
- [86]Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Conscious Cogn 2009;18:176-86. https://doi.org/10.1016/j.concog.2008.12.008
- [87]Hodgins HS, Adair KC. Attentional processes and meditation. Conscious Cogn 2010;19:872-8. https://doi.org/10.1016/j.concog.2010.04.002
- [88]Detweiler MB, Sharma T, Detweiler JG, Murphy PF, Lane S, Carman J, et al. What is the evidence to support the use of therapeutic gardens for the elderly? Psychiatry Investig 2012;9:100-10. https://dx.doi.org/10.4306%2Fpi.2012.9.2.100
- [89]Yewon Cho, Jan Hassink, Vaandrager L. Exploring the Development of Care Farming in South Korea. Korean Society for Agricultural and Food Policy 2019;46:420-43. http://doi.org/10.30805/KJAMP.2019.46.3.420
- [90]de Bruin SR, Pedersen I, Eriksen S, Hassink J, Vaandrager L, Patil GG. Care Farming for People with Dementia; What Can Healthcare Leaders Learn from This Innovative Care Concept? J Healthc Leadersh 2020;12:11-8. https://doi.org/10.2147/jhl.s202988
- [91]Tamilselvam K, Nataraj J, Janakiraman U, Manivasagam T, Essa MM. Antioxidant and anti-inflammatory potential of hesperidin against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced experimental Parkinson’s disease in mice. Int J Nutr Pharmacol Neurol Dis 2013;3:294. https://www.ijnpnd.com/text.asp?2013/3/3/294/114875
- [92]Tamilselvam K, Nataraj J, Janakiraman U, Manivasagam T, Essa MM. Antioxidant and anti-inflammatory potential of hesperidin against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced experimental Parkinson’s disease in mice. Int J Nutr Pharmacol Neurol Dis 2013;3:294. https://www.ijnpnd.com/text.asp?2013/3/3/294/114875