Mucormycosis (black fungus) and its impact on the COVID-19 patients: An updated review
Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global pandemic of the century. The disease is wreaking havoc on human health, the world economy, society, and the environment. It has already caused the loss of millions of lives. Because of the mutation, the virus is constantly evolving itself, changing its nature including the disease transmission rate, virulence, pathogenesis, and clinical manifestations. It was recently reported that certain COVID-19 patients are also suffering from a fungal infection as co-infection commonly known as mucormycosis (black fungus). In India, the outbreak of black fungus in COVID-19 patients has already been declared an epidemic. Only a few reports are noticed in other countries. The focus must now be put toward better management and control of the COVID-19-associated fungal infection. In this review, we have discussed various aspects of black fungus particularly the etiology, taxonomy, risk factors, transmission, pathogenesis, clinical manifestations, diagnosis, and line of treatment to keep up to date on how to manage this fungal infection better.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a serious ongoing public health problem throughout the world. Within a short period of time, the COVID-19 was declared a pandemic by the World Health Organization (WHO) [1]. The disease has become more hazardous due to its zoonotic nature [2, 3]. The COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which is an extremely contagious RNA virus that affects the respiratory systems and associated organs including the heart, kidneys, and the brain [4]. The patients have a variety of clinical symptoms, and the majority of the complications are associated with respiratory organs such as pneumonia, dyspnea, and hypoxia, followed by respiratory failure, septic shock, and multi-organ dysfunction [5]. Other consequence includes diffuse alveolar damage which causes acute respiratory distress syndrome, disseminated intravascular coagulation depletion of white pulp in the spleen, etc. [6]. SARS-CoV-2 can affect people of any age, although a severe form of the disease has been reported predominantly in the elderly and those with additional comorbidities such as cardiovascular diseases, asthma, autoimmune diseases, gastrointestinal diseases, hypertension, immunosuppression, metabolic and neurologic diseases, obesity, renal diseases, etc. [7-10]. COVID-19 patients with comorbid conditions may also develop immunosuppression conditions [11]. This immunosuppression can occur for various reasons, but the most important causes are the high dose and long-term usage of corticosteroids in the COVID-19 patients as a therapeutic measure [12, 13]. The COVID-19 patients might be co-infected with other microbial infections depending on the immune status [14].
The majority of the fungal pathogens are opportunistic. Fungal infections have been increasing due to the increased number of immunocompromised hosts and various types of mycoses may develop depending on the immune status of the patients [15]. COVID-19 patients were also shown to have fungal infections such as Aspergillus, Candida, mucormycetes, etc. [16, 17]. Mucormycosis, popularly known as the black fungus, is a fungal infection caused mostly by Mucorales species such as Rhizopus and Mucor [18, 19]. The disease is rare but may be serious around the globe, with a high incidence in India [20, 21]. The majority of invasive fungal infections occur in immunocompromised patients due to the poor health management system [21, 22]. Mucormycosis has been linked to a number of pre-existing health conditions, including diabetes mellitus, iron overload, cancers, organ transplant, kidney failure, co-infection with Mycobacterium tuberculosis, an acquired immunodeficiency syndrome (AIDS), immunosuppressive therapy, and others [23-25]. In addition, COVID-19 patients may develop immunosuppression as well as increased blood glucose levels in both non-diabetic and diabetic cases. Therefore, COVID-19 associated mucormycosis (CAM) have been reported in the post-treated or recovered patients [17]. CAM reports are currently on the rise all over the world, especially in Asiatic regions where the fatality rate among COVID-19 patients is high [17, 26]. However, the information regarding the causal agents of mucormycosis and CAM is limited. Therefore, this review is focused on the taxonomy and genomic characteristics of mucormycosis etiologies, as well as transmission, clinical symptoms, pathogenesis, etc. In addition, we emphasized on pathogenesis and virulence factors of COVID-19/mucormycosis co-infections, as well as their epidemiology, diagnosis, and line of treatment in CAM patients.
ETIOLOGICAL AGENTS AND TAXONOMY
The etiological agents of mucormycosis belong to the order Mucorales under the phylum Glomeromycota and subphylum Mucoromycotina. The disease was described as zygomycosis under the Zygomycota phylum that includes two diseases: mucormycosis and entomophthoromycosis [19]. A comprehensive molecular phylogenetic re-analysis of fungi eliminated the phylum Zygomycota and replaced it with the phylum Glomeromycota that includes four subphyla e.g. Mucoromycotina, Entomophthoromycotina, Zoopagomycotina, and Kickxellales [27, 28]. In addition, the analysis showed that the subphyla Mucoromycotina and Entomophthoromycotina are evidently unconnected with separate clades. Therefore, the term zygomycosis has become obsolete [28]. Furthermore, the etiological agents are able to generate the word “Mucormycosis” over “Zygomycosis” due to their differences in morphology, epidemiology, clinical symptoms, and ecology [28].
Out of the 261 species in the Mucorales order, 38 are linked to human diseases [29]. Molecular phylogenetic researches have aided in the changing of their taxonomy in earlier years, and numerous taxa have seen repeated name modifications, e.g. (1) Rhizopus oryzae has changed to Rhizopus arrhizus, (2) from Rhizopus microsporus var. azygosporus, var. chinensis, var. oligosporus, var. rhizopodiformis, var. tuberosus to Rhizopus microsporus, (3) from Mucor ellipsoideus, Mucor circinelloides f. circinelloides to Mucor ardhlaengiktus, (4) from Mucor circinelloides f. griseocyanus to Mucor griseocyanus, (5) from Rhizomucor regularior, Rhizomucor variabilis var. regularior to Mucor circinelloides, (6) from Rhizomucor variabilis to Mucor irregularis, (7) from Mucor circinelloides f. lusitanicus to Mucor lusitanicus, (8) from Mucor circinelloides f. janssenii to Mucor janssenii, (9) from Absidia corymbifera, Mycocladus corymbifer to Lichtheimia corymbifera, (10) from Absidia ornata to Lichtheimia ornat, (11) from Absidia ramosa, Mycocladus ramosus, etc. [29, 30].
The common etiological agents of mucormycosis are Mucor spp., Rhizopus spp., and Lichtheimia spp., accounting for about 75% of all cases. Other genera including Rhizomucor, Cunninghamella, Saksenaea, Apophysomyces, Cokeromyces, Syncephalastrum, and Actinomucor are also causative agents of mucormycosis. However, their impacts on the cause of mucormycosis are minor [30]. The causative agents of mucormycosis may be different due to differences in the geographical distribution, e.g. in Europe, Rhizopus spp. were found in 34% of the cases, both Mucor spp. and Lichtheimia spp. were in 19% of the cases; in France, 52% cases were due to Rhizopus spp., and 29% cases due to Rhizopus spp.; in Mexico, Apophysomyces mexicanus was found as a common causative agent for mucormycosis; in India and Asia, Rhizopus spp. is the most common etiological agent [30-32]. Several emerging and uncommon species were also the causative agents for mucormycosis including Apophysomyces elegans, A. variabilis, Rhizopus homothallicus, Thamnostylum lucknowense, Mucor irregularis, and Saksenaea spp. [33-37]. Because of their fast-growing and thermo-tolerant qualities, as well as their ability to grow on decaying organic matter or agricultural and forest soils, these organisms are found all over the world [38].
RISK FACTORS
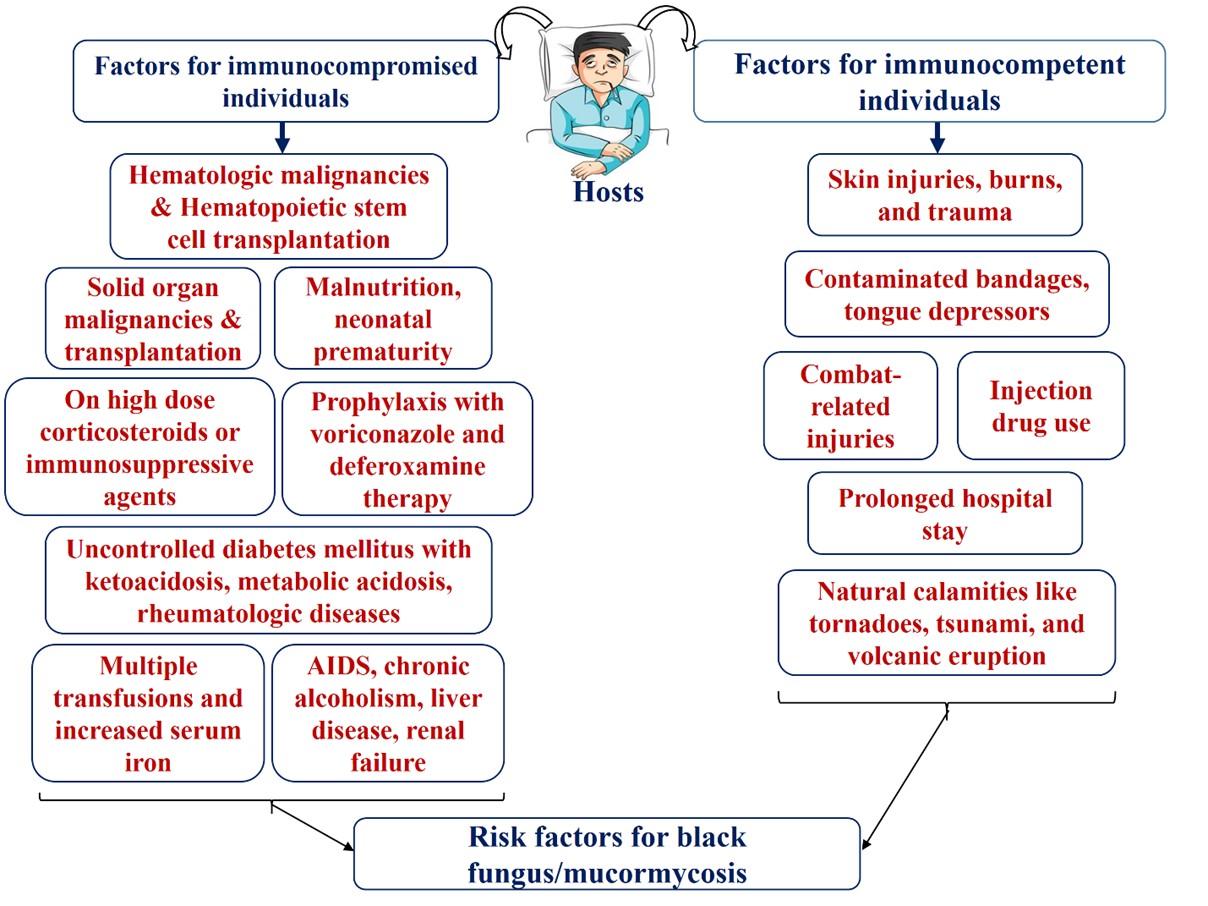
Mucormycosis is an opportunistic disease that has adverse consequences on immunocompromised individuals who have certain risk factors. However, immunocompetent hosts are also susceptible to mucormycosis with a very small percentage [34, 39]. The risk factors associated with mucormycosis weaken the hosts’ immune system, strengthen the growth of the agents, and permit their dissemination to the environment where they can cause significant invasive infections. Figure 1 illustrates the most major risk factors associated with mucormycosis.
Like etiological agents, risk factors of mucormycosis vary with the geographical areas e.g. hematological malignancy is the most common risk factor in Europe, and diabetes mellitus in India, Mexico, Iran, and different countries of the Middle East and North Africa [30, 31, 34, 40-45].
TRANSMISSION AND CLINICAL PRESENTATION OF MUCORMYCOSIS
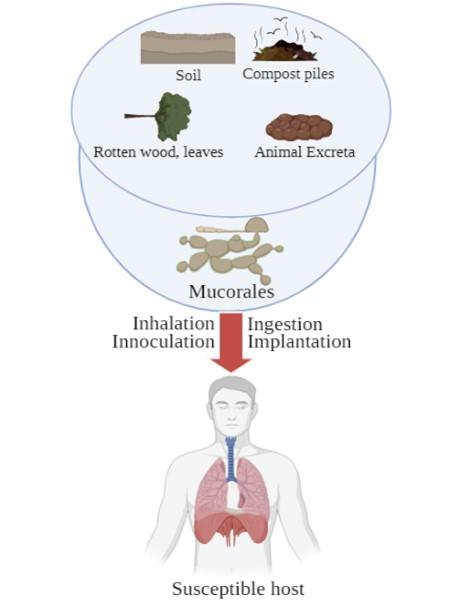
Mucorales, also known as thermotolerant molds, are found widely in the environment, particularly in soil and decaying organic matter such as compost piles, leaves, rotten wood, and animal excreta. Mucorales sporangiospores with a diameter of 3-11 μm can easily be aerosolized and dispersed throughout the environment. Because the spores are airborne, they can be found on any human surface that comes into touch with air, and this is the primary mechanism of transmission. In addition, soil to skin penetration through any burned, cut, or wounded skin and another transmission mode like ingestion by contaminated food are also possibilities. Insects such as stings or bites can also take part in the transmission of fungal agents. Finally, nosocomial transmission occurs often via various transmissions route [46]. The transmission of mucormycosis to humans is illustrated in Figure 2.
Mucormycosis is known to be a threat for immunocompromised and immunocompetent patients (Figure 1). The symptoms of infection are mainly depended on the fungus growing site in the body. Mucormycosis has five major clinical presentations based on organ involvement: rhinocerebral (sinus and brain), pulmonary (lungs), cutaneous (skin), gastrointestinal (GI), disseminated, and rare conditions including kidney infection, osteomyelitis, peritonitis, and endocarditis [47, 48].
Rhinocerebral Mucormycosis
A disease condition caused by Mucormycetes usually infect sinuses and can extend to the brain is called rhinocerebral mucormycosis. This is the most common among all forms of mucormycosis in patients having diabetes mellitus with ketoacidosis (70%) [49]. Rhinocerebral mucormycosis is rare but the disease may occur in patients who receive hematopoietic stem cells or solid organ transplants like kidneys [50]. Germination begins after inhalation of fungal spores into the paranasal sinuses, and it quickly spreads to the surrounding tissues. The initial sign is usually bloody nasal discharge [51]. Due to the disease, inflammation of the sinus and periorbital skin tissues occurs and can cause facial pain and numbness, blur or double vision, eyelid edema, and even vision loss. Because the fungus extends from sinuses to mouth, black, necrotic ulcers with pain in the hard palate are particularly prevalent. Moreover, the hallmark of rhinocerebral mucormycosis is the black, necrotic eschar on the face, however, the absence of this mark does not exclude the possibility. Fever and headache are common, but fever may not be found in up to 50% of cases. An elevated white blood cell (WBC) count is observed as long as the patient has active bone marrow. When the fungus reaches into the brain, partial paralysis, slurred speech, brain abscess, altered consciousness, and coma may be found [49].
Pulmonary Mucormycosis
Mucormycosis associated with the lungs is known as pulmonary mucormycosis. This form of mucormycosis is more common in leukemic patients who are receiving chemotherapy treatment and in hematopoietic stem cell transplant patients who have neutropenia [52]. Inhalation, hematogenous, and/or lymphatic dissemination are the most common causes of this infection. Because it is confused with pulmonary aspergillosis, the symptoms of pulmonary mucormycosis symptoms are nonspecific. Patients, on the other hand, frequently come to health care with high fever (380C) and coughs that are resistant to broad-spectrum antibiotics. Chest pain, dyspnea, and hemoptysis are also present, but they are less common. Rarely, the infection might manifest as tracheal or endobronchial lesions, which can cause airways blockage, lungs collapse, and massive hemoptysis in diabetes patients. Besides lungs, the fungus may also invade the mediastinum, pericardium, and chest wall [53-55].
Cutaneous Mucormycosis
Immunosuppressed patients with a lack of normal protective skin barrier are at high risk of cutaneous mucormycosis. Typically, mucormycosis agents are incapable of penetrating the intact skin; however, if a burn or wound is present, the organism can easily infiltrate the skin and infect dipper tissues [50]. Catheter insertion and insulin injection sites increase the risk of cutaneous mucormycosis in immunocompromised diabetes patients. Mucormycosis agents are abundantly distributed in the soil, hence soil-associated penetration is common. Following penetration, the infection spreads to all the layers of the skin, including the fat and muscles, resulting in gangrene and hematogenous dissemination [56-58]. Clinical presentations include inflammatory reactions with tissue swelling, abscess formation, pus, and necrosis leading to tissue slough-off and large ulcer. Black eschars are often formed with the progression of the ulcer. Moreover, it has the potential to mimic gangrenous pyoderma and other bacterial or fungal infections [59]. Aerial mycelium is occasionally visible to the naked eye on cutaneous lesions [51].
Gastrointestinal (GI) Mucormycosis
Adults are less likely to develop GI mucormycosis, which is more common in preterm neonates and malnourished children. It is also found in patients with diabetes mellitus and a history of corticosteroid treatment [50]. The disease is promoted by spore ingestion through contaminated foods such as fermented milk, dried bread, and corn-origin alcohol [60, 61]. Furthermore, a scientist group reported healthcare-associated ingestion of sporangiospores during oropharyngeal examinations by tongue depressors [62]. The infection may occur in any part of the GI system, but the stomach, colon, and ileum are the most commonly affected. Clinical symptoms are nonspecific and vary depending on which part of the primary gastrointestinal tract is affected. Common symptoms include distended abdomen with pain, nausea, vomiting, and gastrointestinal bleeding. Patients often feel to have an intra-abdominal abscess. Fever and hematochezia can also be found [49].
Disseminated Mucormycosis
Mucormycosis that disseminate hematogenously from the primary site of infection is called disseminated mucormycosis. Lungs are the most common source of mucormycosis-related dissemination. Dissemination can also occur from the GI tract, sinus, and skin. The brain, on the other hand, is the most frequent site of dissemination, followed by the spleen, heart, skin, and other organs. Rhinocerebral mucormycosis particularly, brain to cerebral dissemination is very common. Although disseminated mucormycosis has a mortality record of up to 100% if there is any involvement of the brain. The symptoms of this infection vary widely, as it is associated with the host, organ, and degree of vascular invasion [49, 50].
MORTALITY/CASE FATALITY RATE
Mucormycosis is a rare, but frequently deadly infection. The case fatality rate (CFR) of mucormycosis depends on different underlying locations and conditions, and the type of the fungus. Patients with disseminated infection and haematologic malignancies have a greater CFR of mucormycosis; also, patients on antifungal medication alone have a higher risk [63]. Since the disease is very rare, the determination of the exact data of morbidity and mortality rate of mucormycosis is difficult. However, previous several studies showed different data on CFR of mucormycosis (Table 1).
Table 1. Mortality or case fatality rate of mucormycosis in different case studies around the globe.
PATHOGENESIS AND GENETIC VIRULENCE FACTORS
Because the Mucorales fungus is so common in the environment, such as dirt and decomposing waste, humans are constantly exposed to it [68]. However, the disease-producing efficiency of these fungi is rare, as most of them are less virulent [68]. The mucormycosis may cause death in immunocompromised patients having diabetic mellitus, receiving glucocorticoids and organ transplantation, showing neutropenia, hyperglycemia, skin burning, and/or elevated serum iron level [38, 69]. The fungal spores firstly evade the host’s innate immune system after successful entry either through inhalation, ingestion, or traumatic skin, followed by occurring germination, angioinvasion, and tissue necrosis [68, 69]. Depending on the route of infection, mucormycosis lesions may develop at sites such a lung alveolus, eyes, gastrointestinal tract, cutaneous, etc. Spore coat protein homologs (CotH) of Mucorales can express on the fungal surface and interact with the host cellular receptor named glucose-regulated protein 78 (GRP78) of the endothelium. This phenomenon assists to intervene in the host cell invasion and thereby succeeding angioinvasion [68, 70]. Mucorales then down-regulated several host defense, pathogen identification, and tissue repair genes, resulting in the lethal mucormycosis [28, 68].
High-affinity iron permease (FTR1) of Rhizopus oryzae is an important virulent factor because it expresses during the infection in diabetic ketoacidosis (DKA) mice [71, 72]. Fungal alkaline Rhizopus protease enzyme (Arp) plays an important role to enhance the coagulation process of mucormycosis patients [73]. ADP-ribosylation factor (Arf) and calcineurin (CaN) are required for fungal growth and structural alterations respectively, and hence operate as virulent factors in mucormycosis-causing species [74, 75].
PATHOLOGIES AND OUTCOMES OF COVID-19 AND MUCORMYCOSIS CO-INFECTION
COVID-19, caused by the SARS-CoV-2, is a severe viral pandemic disease of international concern throughout the world, affecting people of all ages [76]. Patients with comorbid conditions, immunosuppression, and immunodeficiency are at higher risk of developing a more severe form of COVID-19 [77]. Patients with the severe form of COVID-19 may require hospitalization, intensive care, and breathing support, followed by recovery or death [78]. There are various kinds of comorbidities associated with COVID-19 severity such as diabetes, chronic kidney disease, chronic lung diseases, chronic obstructive pulmonary disease, asthma, interstitial lung disease, cystic fibrosis, pulmonary hypertension, heart failure, coronary artery disease, cardiomyopathies, hypertension, HIV infection, thalassemia, cancer, cerebrovascular diseases, thalassemia, etc. [79-81]. Patients’ immune systems may be harmed as a result of these medical problems. In addition, most fungal infections are associated with the health and immunosuppressive conditions of the patients [15, 78, 82]. The pathogenesis of CAM is audited in Figure 3.
Various studies reported that around 5% of COVID-19 associated co-infections are fungal, such as Aspergillus and Candida [16, 83]. Although COVID-19 associated mucormycosis (CAM) is rare, when it does occur, it can cause serious consequences in patients [17]. According to recent case reports and clinical data, most of the CAM patients had diabetes mellitus, hypertension, asthma, obesity, hypothyroidism, ischemic cardiomyopathy, or end-stage renal disease [17]. In addition, Garg et al. [17] analyzed a total of nine cases with CAM and found that only two patients were alive and the other seven were died due to various complications. Several vital organs of the patients such as lungs, heart pericardium, brain, kidney, rhino-orbito-cerebral, etc. were affected during the CAM [17, 84, 85]. A 41-year-old COVID-19 patient with a history of type 1 diabetic mellitus (T1DM) was diagnosed with rhinocerebral mucormycosis [86], however, the patient was able to recover after receiving proper treatment. A recent systematic review on 101 cases of CAM showed that around 80% of patients were suffering from diabetes mellitus followed by diabetic ketoacidosis (DKA) (~15%) [87]. Surprisingly, most of the CAM patients (76%) have been treated with corticosteroids [87]. In addition, high dosages and long-time use of corticosteroid treatment cause significant immunosuppression in the patients [88]. The COVID-19 patients developed mucormycosis about two weeks after being admitted to the hospital, regardless of their age [17]. A clinical study in India found that 16 of the 18 COVID-19 patients were using steroids, 16 had diabetes, and 16 had mucormycosis [89]. Similar types of findings have been published by different research groups [90, 91]. Therefore, COVID-19 patients treated with high dosages of corticosteroids for a long time might be a predisposing factor for the development of CAM. The overall scenarios of several CAM cases are documented in Table 2.
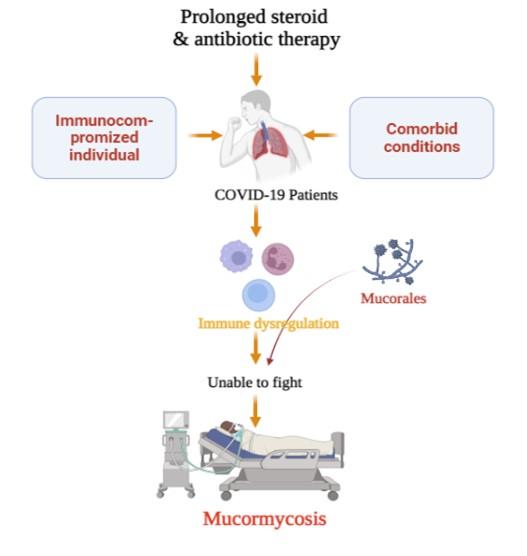
Table 2. Comorbidities, major clinical pathologies, and final outcomes of various COVID-19 associated mucormycosis (CAM) patients.
COUNTRIES AFFECTED BY COVID-19 ASSOCIATED MUCORMYCOSIS
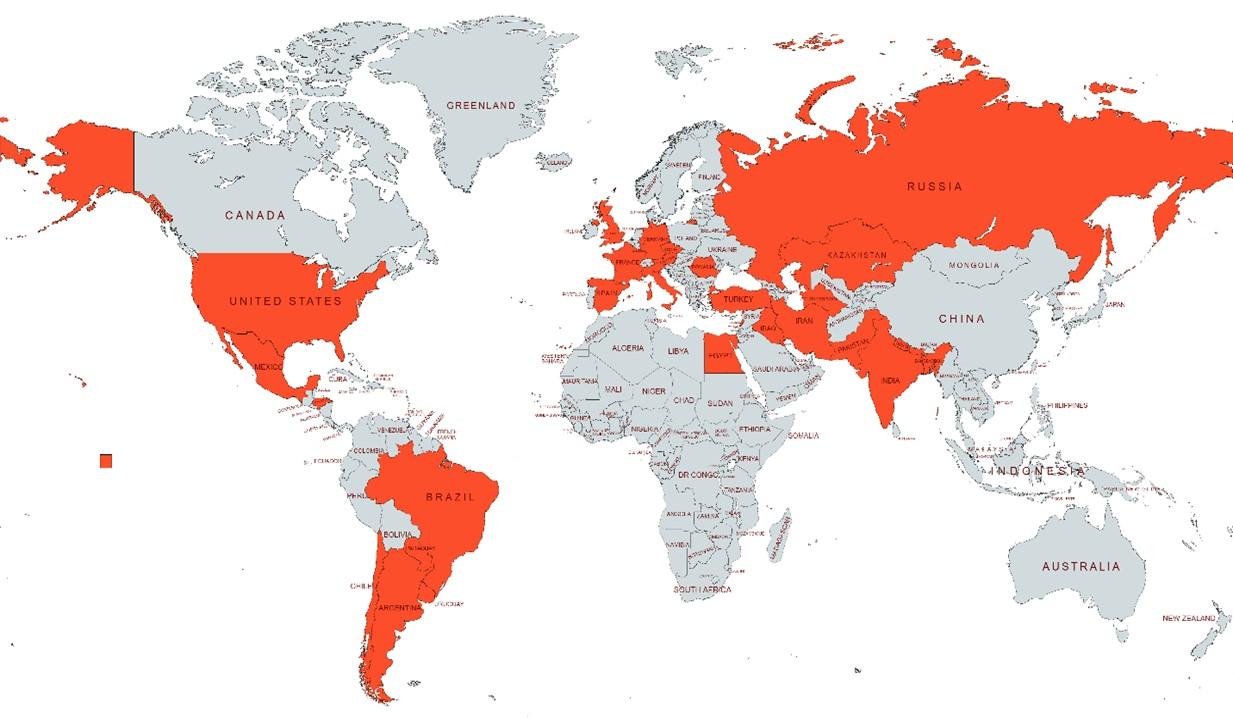
COVID-19 associated mucormycosis (CAM) and other fungal co-infections are on the rise all over the world [17]. Currently, CAM is significantly higher in India compared to developed countries [87]. However, recently published data showed that CAM has been also reported in the USA, UK, Brazil, Italy, France, Iran, Turkey, Mexico, Austria (Figure 4) [17, 87]. Though there is no scientific publication, the various international and national reputed newspapers reported the CAM in Bangladesh (Figure 4) [100].
DIAGNOSIS
Rapid and accurate diagnosis of mucormycosis is always challenging. An experienced physician can diagnose the cases of mucormycosis based on clinical findings and the patient’s history of immunosuppression resulting from medical conditions such as hematological malignancies, uncontrolled diabetes, chemotherapy, etc. Radiologically showing multiple (≥10) nodules accompanying pleural effusion are frequently found to be liked with pulmonary mucormycosis. Computerized tomography (CT) scan is also used for observing such lesions of the respiratory organs. Among the CT scan, the positron emission tomography-computed tomography (PET/CT) aided with [18F]-fluorodeoxyglucose (FDG) is one of the most common tools [72, 101].
Isolation and identification of Mucorales by culture are well-established protocols for the diagnosis of mucormycosis. However, a fungal culture is far more difficult to cultivate than bacterial culture and may not always be effective. Because fungal hyphae are particularly friable in nature and readily injured during tissue manipulation, inoculum production from tissue samples needs to be gentle [102]. When clinical samples are utilized, it may take 3 to 7 days or more to observe fungal colonies on a fungal medium such as Sabouraud dextrose agar or potato dextrose agar, which are commonly treated with antibiotics to reduce contaminated bacterial development. Culture plates are usually incubated at 25°C to 30°C under aerobic conditions and in the presence of moisture. The slide culture technique is a reliable method to culture fungi that allow studying colonies without changing their morphology too much [51].
Direct microscopy and histopathological study of the affected tissues is also useful technique for the diagnosis of the disease. Tissue stains such as H&E (Hematoxylin and eosin) stain, PAS (Periodic acid-Schiff), or Grocott-Gomori’s methenamine silver staining are commonly used to observe fungal hyphae and morphology under the microscope [103].
Serological tests have high diagnostic values in disease diagnosis. In the serological test, either antigen or antibody has to be known to identify the unknown. Despite the possibility of cross-reaction in some of the serological tests, these tests are reliable diagnostic tools. Serological tests such as enzyme-linked immunosorbent assay (ELISA), immunoblotting, and immunodiffusion tests are available for the detection of fungal antigen or antibodies responsible for mucormycosis [104-106].
Confirmatory diagnosis is based on agent identification using fungal culture, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, polymerase chain reaction (PCR), restriction fragment length polymorphism (RFLP), and DNA/genome sequencing [102, 107]. The internal transcribed spacer or the 18S rRNA genes specific for the etiology of mucormycosis are usually targeted by PCR (standard PXR, real-time PCR, and quantitative PCR/qPCR) [108-110]. In addition, spore coat proteins encoding gene (cotH) has also been used as the target gene for the detection of Mucorales [111]. Many of these PCR has been successfully applied for the conform diagnosis of mucormycosis. Experimental findings suggest that PCR is capable to detect Mucorales from fresh and formalin-fixed clinical samples, blood, serum, etc. [112, 113]. However, further optimization of these protocols is underway to validate their application for routine diagnostic purposes [114].
LINE OF TREATMENT
After more than a year since the first appearance of the novel coronavirus infection, a novel, definite and effective therapy is yet to be developed. Despite the recent development of vaccines which are still undergoing various phases of clinical trials to determine their efficacy and safety, many therapeutic agents are being tested including antiviral drugs used in other viral infections. The current treatments are mostly symptomatic and for associated complications. Therefore, treating COVID-19 patients with weak immunity and comorbidity remains challenging [115]. Due to the associated comorbidity and immune-compromised conditions, patients are susceptible to grow severe deceitful infections such as different fungal infections [116, 117]. Recent cases of mucormycosis infection in COVID-19 patients are like to slay the slain. The treatment strategies for CAM have been recorded in a number of case reports published recently. Currently, two main treatment strategies are followed to treat this deadly fungal co-infection: (1) medication and (2) surgery, in addition to continued antiviral and symptomatic treatments of SARS-CoV-2 disease.
When compared to drugs/medicines available to treat bacterial or other fungal diseases, mucormycosis has a limited number of treatment options. Polyenes and azoles are the only two groups of compounds being employed in therapeutic practice. Furthermore, because mucormycosis is a rare disease with a wide range of hosts and infection sites, as well as a wide range of offending Mucorales, it is difficult to obtain reliable therapy data [118].
The basis of mucormycosis treatment of COVID-19 patients
The mucormycosis treatment strategy incorporates either simultaneous application of multiple interventions or with different timing and intensity. The main steps of mucormycosis therapies include the assessment of disease severity and aggressive clinical and laboratory diagnosis, timely initiation of effective antifungal therapy (monotherapy and combination therapy) alongside intense surgical debridement of skin lesions, and immunosuppression reversal by withdrawing chemotherapy and immunosuppressive drugs [118]. Rapid diagnosis and early therapeutic intervention are critical to avoid progressive tissue invasion which ultimately might decrease the need for extensive surgical intervention to improve survival [119].
Antifungal mono or combination therapy
Due to high morbidity and mortality, quick and aggressive administration of antifungal drugs is usually given. A number of drugs choice are available including amphotericin B, voriconazole, caspofungin, isavuconazole, posaconazole, echinocandin, anidulafungin, nystatin, etc. After reviewing 80 individual mucormycosis infections of COVID-19 patients extracted from different case reports published until May 2021, amphotericin B and its lipid complex formulation were predominantly used [17, 84, 120-124]. Besides, isavuconazole and voriconazole have also recently been considered as primary drugs of choice depending on the types of molds. However, according to the case reports (12/17), this drug was used mostly in either dual or triple combination with other mold-active antifungals such as amphotericin B, caspofungin, isavuconazole, and anidulafungin [121]. Posaconazole is used as rescue therapy when the first-line drugs do not respond.
In the treatment of mucormycosis, amphotericin B is the key drug of choice [118]. Lipid formulations of amphotericin B have a greater therapeutic effect over the conventional amphotericin B deoxycholate [125, 126]. The fungicidal and fungistatic effect of amphotericin B is concentration-dependent. It binds to fungal cellular ergosterol and alters membrane permeability and the leakage of cellular components [127]. Posaconazole is a second-line treatment for patients who are intolerant to amphotericin B or who require long-term maintenance medication [128]. Because experimental findings show that posaconazole can cause a breakthrough infection when administered as a prophylactic, it is not recommended as a first-line mucormycosis treatment [129, 130]. Isavuconazole also showed potent activity against mucormycosis agents and received approval for therapy. Posaconazole and isavuconazole both suppress fungal ergosterol production. Caspofungin and anidulafungin are cyclic lipopeptide Echninocandins group of antifungals that have specific activity against mucormycosis when used in combination therapy. They inhibit the β-D-glucan synthase presented in R. oryzae and damage the fungal cell wall [131].
The use of a combination of antifungals in severely immunocompromised individuals is becoming more widespread due to synergistic effects and larger coverage. However, antagonism, drug-drug interaction, toxicity, and expense are the drawbacks [132]. An in vitro experiment showed that anidulafungin and posaconazole had a synergistic effect on Aspergillus fumigatus infection, with enhanced survival and lower fungal load when compared with their individual effects [133]. Moreover, the isavuconazole-micafungin combination exhibited synergistic activity against Aspergillus species ex vivo [134]. Furthermore, statins such as lovastatin exhibited anti Rhizopus spp. activity both in vitro and in vivo when combined with unreliable limited clinical evidence [120, 135].
Adjunctive therapy and surgery
It is well established that immunosuppressive patients are more prone to mucormycosis infection. Patients with COVID-19 immunity who also have diabetes mellitus are widely challenged. In addition to this, the use of steroids in moderate to severe COVID-19 patients further imposes immunosuppression. Immunosuppression setback strategy could be noteworthy for mucormycosis. Corticosteroid-mediated immunosuppression in COVID-19 patients should be lowered or switched to an alternative non-steroidal medication [118]. For individuals with uncontrolled diabetes and/or ketoacidosis, rapid glycemic management is critical. Sodium bicarbonate treated reversal of acidemia reasonably disabled the invasion ability of R. oryzae into the endothelial cells [136]. Increased iron assimilation and serum levels cause enhanced growth of Mucorales and their pathogenesis as confirmed by in vitro and pre-clinical, and retrospective human studies [137, 138]. Therefore, iron chelators have been recommended as a possible supplementary treatment by researchers. Preclinical investigations demonstrated that when mice were administered deferasirox, they lived longer [139].
On the other hand, a large number of populations worldwide take zinc tablets to enhance their immune system and fight COVID-19. A recent report states that the sales of zinc supplements jumped by 93% in India during 2020. Zinc’s importance in the therapy of COVID-19 has been related to its immunomodulatory properties, antiviral properties, and capacity to control the inflammatory response [140]. A number of studies have been reported that show the effectiveness of zinc therapy in the treatment of COVID-19 patients [141]. However, zinc acquirement is vital during the fungal life cycle for proper development when they are saprophytes or even during the infection process [142]. Therefore, zinc chelation is another approach to treat mucormycosis. Study reveals that different zinc chelators such as clioquinol, phenanthroline, and N,N,N′,N′-tetrakis (2-pyridylmethyl) ethane-1,2-diamine in combination with either amphotericin B or posaconazole showed inhibitory effect against 25 Mucorales strains, with the clioquinol-posaconazole combination being the most active [143]. Reduced Zn hinders fungal growth by inhibiting the activity of Zn-binding transcription factors which are involved in fungal biological processes [142].
Treatment with granulocyte (macrophage) colony-stimulating factor or interferon- has been proposed as adjunct therapy [144, 145]. In a recent case report, combined therapy of interferon-ϒ and nivolumab successfully treated an intractable mucormycosis in an immunodeficient, trauma patient [146].
During mucormycosis, blood vessel thrombosis and the resultant tissue necrosis can prevent antifungal medicines from reaching the infection site. As a result, debridement of necrotic tissues may be necessary for full mucormycosis eradication. Patients who did not have their mucormycosis surgically debrided had a far greater death rate than those who did [130].
A special observation to consider in pregnant COVID-19 patients with mucormycosis infection
The acute inflammatory response in COVID-19 causes erythrocytic damage, leading to increased iron loss from the body. This exacerbates iron deficiency anemia and may worsen coronavirus infection [147]. Iron deficiency anemia in pregnancy is common and iron salt supplement is generally given. However, a higher level of iron provides a favorable environment for mucormycosis development. The relationship between the growth of mucormycosis and iron level has been discussed above. Therefore, iron supplementation in pregnant COIVD-19 patients may need careful monitoring. Early iron deficiency anemia diagnostics, which involves measuring serum ferritin and iron levels in COVID-19 positive pregnant women, as well as a proper data interpretation on serum ferritin levels in pregnant women with COVID-19 associated mucormycosis, is important.
New antifungal candidates in the pipeline
The search for new antifungal medication is still ongoing due to the restricted number of available drugs. Recently, a novel glucan synthase inhibitor SCY-078 demonstrated strong fungicidal activity against azole-susceptible strains of A. fumigatus when given alone. The activity was better than amphotericin B and voriconazole. It also exhibited synergy with voriconazole, isavuconazole, and amphotericin B [148]. Another novel fungal CYP 51 inhibitor VT-1161 possess in vitro activity against R. oryzae, Lichtheimia, and Cunninghamella. APX001A (formerly E1210) is an antifungal agent which is under phase I clinical trials protected immunosuppressed mice with R. delemar infection [149]. Lastly, haemofungin hinders in vitro growth of a variety of fungi including Rhizopus [150]. The re-emergence of the CAM epidemic in India will undoubtedly push scientists hard to discover novel antifungal agents.
CONCLUSION AND RECOMMENDATIONS
Mucormycosis might be caused by various species of genus Rhizopus, Mucor and Lichtheimia and its incidence have been increasing recently due to the high number of patients with immunosuppression. There is a strong correlation among pre-existing risk factors for the development of mucormycosis and CAM. The CAM has been becoming an emerging threat to the patients suffering from various comorbid medical conditions such as diabetes and treatment with immunosuppressive drugs such as steroids. Therefore, the COVID-19 patients should be carefully treated with immunosuppressive drugs and if possible, it should be avoided in the cases of pre-existing factors for CAM. Immediate diagnosis and treatment should be started if the COVID-19 patients are suspected of CAM to prevent the severe course of the disease.
ACKNOWLEDGEMENT
None.
AUTHOR CONTRIBUTIONS
Conceptualization, AKMMH, MGH, AMMTR, and MTR; literature collection and curation, AKMMH, MGH, MSI, MAS, and MTR; writing—original draft, AKMMH, MGH, MSI, MAS, and MTR; and writing—review and editing, MGH, MSI, and MTR. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Rahman T, Sobur A, Islam S, Toniolo A, Nazir KN. Is the COVID-19 pandemic masking dengue epidemic in Bangladesh?. Journal of Advanced Veterinary and Animal Research. 2020;7(2):218-219.
- [2]Tazerji SS, Duarte PM, Rahimi P, Shahabinejad F, Dhakal S, Malik YS, et al. Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: an updated review. Journal of Translational Medicine. 2020;18(1):358.
- [3]Rahman M, Sobur M, Islam M, Ievy S, Hossain M, El Zowalaty ME, Rahman AM, Ashour HM. Zoonotic diseases: etiology, impact, and control. Microorganisms. 2020;8(9):1405.
- [4]Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. 2020;5(4):536-544.
- [5]Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497-506.
- [6]Chen W, Pan JY. Anatomical and pathological observation and analysis of SARS and COVID-19: microthrombosis is the main cause of death. Biological Procedures Online. 2021;23(1):1-2.
- [7]Dai SP, Zhao X, Wu JH. Effects of comorbidities on the elderly patients with COVID-19: clinical characteristics of elderly patients infected with COVID-19 from sichuan, China. The Journal of Nutrition, Health & Aging. 2021;25(1):18-24.
- [8]Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people?. Aging (Albany NY). 2020;12(10):9959-9981.
- [9]D’ascanio M, Innammorato M, Pasquariello L, Pizzirusso D, Guerrieri G, Castelli S, et al. Age is not the only risk factor in COVID-19: the role of comorbidities and of long staying in residential care homes. BMC Geriatrics. 2021;21(1):63.
- [10]Islam MS, Sobur MA, Akter M, Nazir KN, Toniolo A, Rahman MT. Coronavirus Disease 2019 (COVID-19) pandemic, lessons to be learned!. Journal of Advanced Veterinary and Animal Research. 2020;7(2):260-280.
- [11]Callender LA, Curran M, Bates SM, Mairesse M, Weigandt J, Betts CJ. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Frontiers in Immunology. 2020;11:1991.
- [12]Schoot TS, Kerckhoffs AP, Hilbrands LB, Van Marum RJ. Immunosuppressive drugs and COVID-19: a review. Frontiers in Pharmacology. 2020;11:1333.
- [13]Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(5):971-978.
- [14]Feldman C, Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia. 2021;13(1):1-5.
- [15]Stein DK, Sugar AM. Fungal infections in the immunocompromised host. Diagnostic Microbiology and Infectious Disease. 1989;12(4):221-228.
- [16]Guo L, Wei D, Zhang X, Wu Y, Li Q, Zhou M, Qu J. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Frontiers in Microbiology. 2019;10:2752.
- [17]Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;86(2):289-298.
- [18]Gomes MZ, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus,-Mucor, and-Lichtheimia species. Clinical Microbiology Reviews. 2011;24(2):411-445.
- [19]Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clinical Infectious Diseases. 2012;54(suppl_1):S8-S15.
- [20]Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. Journal of Fungi. 2020;6(4):265.
- [21]Slavin MA, Chakrabarti A. Opportunistic fungal infections in the Asia-Pacific region. Medical Mycology. 2012;50(1):18-25.
- [22]Badiee P, Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis & clinical management. The Indian Journal of Medical Research. 2014;139(2):195-204.
- [23]Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. Journal of Fungi. 2019;5(1):26.
- [24]Prakash H, Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(3):523.
- [25]Lin E, Moua T, Limper AH. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45(4):443-448.
- [26]Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, et al. The Emergence of COVID-19 Associated Mucormycosis: Analysis of Cases From 18 Countries. 2021. Available at: https://ssrn.com/abstract=3844587.
- [27]Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher-level phylogenetic classification of the Fungi. Mycological Research. 2007;111(5):509-547.
- [28]Binder U, Maurer E, Lass‐Flörl C. Mucormycosis–from the pathogens to the disease. Clinical Microbiology and Infection. 2014;20(suppl_6):60-66.
- [29]Walther G, Wagner L, Kurzai O. Updates on the taxonomy of Mucorales with an emphasis on clinically important taxa. Journal of Fungi. 2019;5(4):106.
- [30]Skiada A, Pagano LI, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clinical Microbiology and Infection. 2011;17(12):1859-1867.
- [31]Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, et al. A global analysis of mucormycosis in France: the RetroZygo Study (2005–2007). Clinical Infectious Diseases. 2012;54(suppl_1):S35-S43.
- [32]Bonifaz A, Stchigel AM, Guarro J, Guevara E, Pintos L, Sanchis M, Cano-Lira JF. Primary cutaneous mucormycosis produced by the new species Apophysomyces mexicanus. Journal of Clinical Microbiology. 2014;52(12):4428-4431.
- [33]Chakrabarti A, Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57(suppl_3):85-90.
- [34]Chakrabarti A, Das A, Mandal J, Shivaprakash MR, George VK, Tarai B, et al. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Sabouraudia. 2006;44(4):335-342.
- [35]Chakrabarti A, Marak RS, Shivaprakash MR, Gupta S, Garg R, Sakhuja V, et al. Cavitary pulmonary zygomycosis caused by Rhizopus homothallicus. Journal of Clinical Microbiology. 2010;48(5):1965-1969.
- [36]Chander J, Stchigel AM, Alastruey-Izquierdo A, Jayant M, Bala K, Rani H, et al. Fungal necrotizing fasciitis, an emerging infectious disease caused by Apophysomyces (Mucorales). Revista Iberoamericana De Micología. 2015;32(2):93-98.
- [37]Prakash H, Ghosh AK, Rudramurthy SM, Paul RA, Gupta S, Negi V, Chakrabarti A. The environmental source of emerging Apophysomyces variabilis infection in India. Medical Mycology. 2016;54(6):567-575.
- [38]Challa S. Mucormycosis: Pathogenesis and pathology. Current Fungal Infection Reports. 2019;13(1):11-20.
- [39]Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clinical Infectious Diseases. 2005;41(5):634-653.
- [40]Pagano L, Valentini CG, Posteraro B, Girmenia C, Ossi C, Pan A, et al. Zygomycosis in Italy: a survey of FIMUA-ECMM (Federazione Italiana di Micopatologia Umana ed Animale and European Confederation of Medical Mycology). Journal of Chemotherapy. 2009;21(3):322-329.
- [41]Prakash H, Ghosh AK, Rudramurthy SM, Singh P, Xess I, Savio J, et al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Medical Mycology. 2019;57(4):395-402.
- [42]Patel A, Kaur H, Xess I, Michael JS, Savio J, Rudramurthy S, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clinical Microbiology and Infection. 2020;26(7):944.e9-944.e15.
- [43]Corzo-León DE, Chora-Hernández LD, Rodríguez-Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Medical Mycology. 2018;56(1):29-43.
- [44]Vaezi A, Moazeni M, Rahimi MT, de Hoog S, Badali H. Mucormycosis in Iran: a systematic review. Mycoses. 2016;59(7):402-415.
- [45]Stemler J, Hamed K, Salmanton‐García J, Rezaei‐Matehkolaei A, Gräfe SK, Sal E, et al. Mucormycosis in the Middle East and North Africa: Analysis of the FungiScope® registry and cases from the literature. Mycoses. 2020;63(10):1060-1068
- [46]Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clinical Microbiology and Infection. 2009;15(Suppl_5):2-9.
- [47]Lopes JO, Pereira DV, Streher LA, Fenalte AA, Alves SH, Benevenga JP. Cutaneous zygomycosis caused byAbsidia corymbifera in a leukemic patient. Mycopathologia. 1995 May 1;130(2):89-92.
- [48]Stas KJ, Louwagie PG, Van Damme BJ, Coosemans W, Waer M, Vanrenterghem YF. Isolated zygomycosis in a bought living unrelated kidney transplant. Transplant International. 1996;9(6):600-602.
- [49]Spellberg B, Edwards Jr J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clinical Microbiology Reviews. 2005;18(3):556-569.
- [50]Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clinical Infectious Diseases. 2012;54(suppl_1):S23-S34.
- [51]Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clinical Microbiology Reviews. 2000;13(2):236-301.
- [52]Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. The Annals of Thoracic Surgery. 1994;57(4):1044-1050.
- [53]Gupta KL, Khullar DK, Behera D, Radotra BD, Sakhuja V. Pulmonary mucormycosis presenting as fatal massive haemoptysis in a renal transplant recipient. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association-European Renal Association. 1998;13(12):3258-3260.
- [54]Kitabayashi A, Hirokawa M, Yamaguchi A, Takatsu H, Miura AB. Invasive pulmonary mucormycosis with rupture of the thoracic aorta. American Journal of Hematology. 1998;58(4):326-329.
- [55]Passamonte PM, Dix JD. Nosocomial pulmonary mucormycosis with fatal massive hemoptysis. The American Journal of the Medical Sciences. 1985;289(2):65-67.
- [56]Hampson FG, Ridgway EJ, Feeley K, Reilly JT. A fatal case of disseminated zygomycosis associated with the use of blood glucose self-monitoring equipment. Journal of Infection. 2005;51(5):e269-e272.
- [57]Hocker TL, Wada DA, Bridges A, el-Azhary R. Disseminated zygomycosis heralded by a subtle cutaneous finding. Dermatology Online Journal. 2010;16(9):3.
- [58]Rubin AI, Grossman ME. Bull’s-eye cutaneous infarct of zygomycosis: a bedside diagnosis confirmed by touch preparation. Journal of the American Academy of Dermatology. 2004;51(6):996-1001.
- [59]Kerr OA, Bong C, Wallis C, Tidman MJ. Primary cutaneous mucormycosis masquerading as pyoderma gangrenosum. British Journal of Dermatology. 2004;150(6):1212-1213.
- [60]Ismail MH, Hodkinson HJ, Setzen G, Sofianos C, Hale MJ. Gastric mucormycosis. Tropical gastroenterology: official journal of the Digestive Diseases Foundation. 1990;11(2):103-105.
- [61]Oliver MR, Van Voorhis WC, Boeckh M, Mattson D, Bowden RA. Hepatic mucormycosis in a bone marrow transplant recipient who ingested naturopathic medicine. Clinical Infectious Diseases. 1996;22(3):521-524.
- [62]Mitchell SJ, Gray J, Morgan ME, Hocking MD, Durbin GM. Nosocomial infection with Rhizopus microsporus in preterm infants: association with wooden tongue depressors. The Lancet. 1996;348(9025):441-443.
- [63]Kara IO, Tasova Y, Uguz A, Sahin B. Mucormycosis‐associated fungal infections in patients with haematologic malignancies. International Journal of Clinical Practice. 2009;63(1):134-139.
- [64]Petrikkos G, Skiada A, Sambatakou H, Toskas A, Vaiopoulos G, Giannopoulou M, Katsilambros N. Mucormycosis: ten-year experience at a tertiary-care center in Greece. European Journal of Clinical Microbiology and Infectious Diseases. 2003;22(12):753-756.
- [65]Bitar D, Van Cauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerging Infectious Diseases. 2009;15(9):1395-1401.
- [66]Saegeman V, Maertens J, Meersseman W, Spriet I, Verbeken E, Lagrou K. Increasing incidence of mucormycosis in University Hospital, Belgium. Emerging Infectious Diseases. 2010;16(9):1456-1458.
- [67]Ambrosioni J, Bouchuiguir-Wafa K, Garbino J. Emerging invasive zygomycosis in a tertiary care center: epidemiology and associated risk factors. International Journal of Infectious Diseases. 2010;14(Suppl_3):e100-e103.
- [68]Petrikkos G, Tsioutis C. Recent advances in the pathogenesis of mucormycoses. Clinical Therapeutics. 2018;40(6):894-902.
- [69]Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clinical Infectious Diseases. 2012;54(suppl_1):S16-S22.
- [70]Gebremariam T, Liu M, Luo G, Bruno V, Phan QT, Waring AJ, et al. CotH3 mediates fungal invasion of host cells during mucormycosis. The Journal of Clinical Investigation. 2014;124(1):237-250.
- [71]Ibrahim AS, Gebremariam T, Lin L, Luo G, Husseiny MI, Skory CD, et al. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Molecular Microbiology. 2010;77(3):587-604.
- [72]Hassan MI, Voigt K. Pathogenicity patterns of mucormycosis: epidemiology, interaction with immune cells and virulence factors. Medical Mycology. 2019;57(Suppl_2):S245-S256.
- [73]Spreer A, Rüchel R, Reichard U. Characterization of an extracellular subtilisin protease of Rhizopus microsporus and evidence for its expression during invasive rhinoorbital mycosis. Medical Mycology. 2006;44(8):723-731.
- [74]Lee SC, Li A, Calo S, Heitman J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathogens. 2013;9(9):e1003625.
- [75]Patiño-Medina JA, Maldonado-Herrera G, Pérez-Arques C, Alejandre-Castañeda V, Reyes-Mares NY, Valle-Maldonado MI, et al. Control of morphology and virulence by ADP-ribosylation factors (Arf) in Mucor circinelloides. Current Genetics. 2018;64(4):853-869.
- [76]Monod M, Blenkinsop A, Xi X, Hebert D, Bershan S, Tietze S, et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371(6536):eabe8372.
- [77]Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: A systematic review and meta-analysis. The Journal of Infection. 2020;81(2):e93-e95.
- [78]Kim L, Garg S, O’Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clinical Infectious Diseases. 2021;72(9):e206-e214.
- [79]Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its impact on patients with COVID-19. SN Comprehensive Clinical Medicine. 2020;2:1069-1076.
- [80]Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. The European Respiratory Journal. 2020;55:5.
- [81]Targher G, Mantovani A, Wang XB, Yan HD, Sun QF, Pan KH, et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes & Metabolism. 2020;46(4):335-337.
- [82]Low CY, Rotstein C. Emerging fungal infections in immunocompromised patients. F1000 Medicine Reports. 2011;3:14.
- [83]Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185(4):599-606.
- [84]Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plastic and Reconstructive Surgery. 2021;37(2):e40-e80.
- [85]Pasero D, Sanna S, Liperi C, Piredda D, Branca GP, Casadio L, et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2021;49(5):1055-1060.
- [86]Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. Journal of Medical Cases. 2021;12(3):85-89.
- [87]Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2021;15(4):102146.
- [88]oussef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheumatic Disease Clinics. 2016;42(1):157-176.
- [89]Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, et al. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids—An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. Journal of Maxillofacial and Oral Surgery. 2021;20:418-425.
- [90]Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. The Journal of Laryngology & Otology. 2021;135(5):442-447.
- [91]Satish D, Joy D, Ross AB. Mucormycosis co-infection associated with global COVID-19: A case series from India. International Journal of Otorhinolaryngology and Head and Neck Surgery. 2021;7:815-820.
- [92]Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet Microbe. 2020;1(6):e245-e253.
- [93]Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. The American Journal of Emergency Medicine. 2021;42:264.e5-264.e8.
- [94]Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9):e10726-e10726.
- [95]do Monte Junior ES, Dos Santos ME, Ribeiro IB, de Oliveira Luz G, Baba ER, Hirsch BS, et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clinical Endoscopy. 2020;53(6):746-749.
- [96]Placik DA, Taylor WL, Wnuk NM. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiology Case Reports. 2020;15(11):2378-2381.
- [97]Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: A case report. International Journal of Surgery Case Reports. 2021;82:105957.
- [98]Saldanha M, Reddy R, Vincent MJ. of the article: paranasal mucormycosis in COVID-19 patient. Indian Journal of Otolaryngology and Head & Neck Surgery. 2021;22:1-4.
- [99]Revannavar SM, Supriya PS, Samaga L, Vineeth VK. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world?. BMJ Case Reports CP. 2021;14(4):e241663.
- [100]Rahman MT, Hossain MG, Rahman AT, Huq AM, Farzana S, Nazir KN. Mucormycosis (black fungus) in COVID-19 patients—Will it be another matter of concern in the midst of the COVID-19 flare-up in Bangladesh?. Journal of Advanced Veterinary and Animal Research. 2021;8(3):367-369.
- [101]Liu Y, Wu H, Huang F, Fan Z, Xu B. Utility of 18F-FDG PET/CT in diagnosis and management of mucormycosis. Clinical Nuclear Medicine. 2013;38(9):e370-e371.
- [102]Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Medical Mycology. 2018;56(suppl_1):S93-S101.
- [103]Lass‐Flörl C. Zygomycosis: conventional laboratory diagnosis. Clinical Microbiology and Infection. 2009;15(suppl_5):60-65.
- [104]Sandven PE, EDUARD W. Detection and quantitation of antibodies against Rhizopus by enzyme‐linked immunosorbent assay. APMIS. 1992;100(11):981-987.
- [105]Wysong DR, Waldorf AR. Electrophoretic and immunoblot analyses of Rhizopus arrhizus antigens. Journal of Clinical Microbiology. 1987;25(2):358-363.
- [106]Jones KW, Kaufman L. Development and evaluation of an immunodiffusion test for diagnosis of systemic zygomycosis (mucormycosis): preliminary report. Journal of Clinical Microbiology. 1978;7(1):97-101.
- [107]Nyilasi I, Papp T, Csernetics Á, Krizsán K, Nagy E, Vágvölgyi C. High‐affinity iron permease (FTR1) gene sequence‐based molecular identification of clinically important Zygomycetes. Clinical Microbiology and Infection. 2008;14(4):393-397.
- [108]Scherer E, Iriart X, Bellanger AP, Dupont D, Guitard J, Gabriel F, et al. Quantitative PCR (qPCR) detection of Mucorales DNA in bronchoalveolar lavage fluid to diagnose pulmonary mucormycosis. Journal of Clinical Microbiology. 2018;56(8):e00289-18.
- [109]Alvarez E, Sutton DA, Cano J, Fothergill AW, Stchigel A, Rinaldi MG, Guarro J. Spectrum of zygomycete species identified in clinically significant specimens in the United States. Journal of Clinical Microbiology. 2009;47(6):1650-1656.
- [110]Hsiao CR, Huang L, Bouchara JP, Barton R, Li HC, Chang TC. Identification of medically important molds by an oligonucleotide array. Journal of Clinical Microbiology. 2005;43(8):3760-3768.
- [111]Baldin C, Soliman SS, Jeon HH, Alkhazraji S, Gebremariam T, Gu Y, et al. PCR-based approach targeting Mucorales-specific gene family for diagnosis of mucormycosis. Journal of Clinical Microbiology. 2018;56(10):e00746-18.
- [112]Ino K, Nakase K, Nakamura A, Nakamori Y, Sugawara Y, Miyazaki K, et al. Management of pulmonary mucormycosis based on a polymerase chain reaction (PCR) diagnosis in patients with hematologic malignancies: a report of four cases. Internal Medicine. 2017;56(6):707-711.
- [113]Millon L, Herbrecht R, Grenouillet F, Morio F, Alanio A, Letscher-Bru V, et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clinical Microbiology and Infection. 2016;22(9):810.e1-810.e8.
- [114]Voigt K, Cigelnik E, O’donnell K. Phylogeny and PCR identification of clinically important Zygomycetes based on nuclear ribosomal-DNA sequence data. Journal of Clinical Microbiology. 1999;37(12):3957-3964.
- [115]Majid S, Khan MS, Rashid S, Niyaz A, Farooq R, Bhat SA, et al. COVID-19: Diagnostics, therapeutic advances, and vaccine development. Current Clinical Microbiology Reports. 2021; 8:152-166.
- [116]Salehi M, Ahmadikia K, Badali H, Khodavaisy S. Opportunistic fungal infections in the epidemic area of COVID-19: a clinical and diagnostic perspective from Iran. Mycopathologia. 2020;185(4):607-611.
- [117]Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerging Infectious Diseases. 2020;26(11):2694-2696.
- [118]Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. Journal of Fungi. 2018;4:3.
- [119]Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clinical Infectious Diseases. 2012;54(suppl_1):S55-S60.
- [120]Bellanger AP, Tatara AM, Shirazi F, Gebremariam T, Albert ND, Lewis RE, et al. Statin concentrations below the minimum inhibitory concentration attenuate the virulence of Rhizopus oryzae. The Journal of Infectious Diseases. 2016;214(1):114-121.
- [121]Chong WH, Neu KP. The incidence, diagnosis, and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): a systematic review. Journal of Hospital Infection. 2021;113:115-129.
- [122]Nehara HR, Puri I, Singhal V, Sunil IH, Bishnoi BR, Sirohi P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian Journal of Medical Microbiology. 2021;39(3):380-383.
- [123]Khatri A, Chang KM, Berlinrut I, Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient–case report and review of literature. Journal of Medical Mycology. 2021;31(2):101125.
- [124]Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian Journal of Ophthalmology. 2021;69(6):1563-1568.
- [125]Cornely O, Arikan‐Akdagli SE, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clinical Microbiology and Infection. 2014;20(suppl_3):5-26.
- [126]Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102(3):433-444.
- [127]Haidar G, Singh N. How we approach combination antifungal therapy for invasive aspergillosis and mucormycosis in transplant recipients. Transplantation. 2018;102(11):1815-1823.
- [128]Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013;98(4):492-504.
- [129]Lekakis LJ, Lawson A, Prante J, Ribes J, Davis GJ, Monohan G, et al. Fatal rhizopus pneumonia in allogeneic stem cell transplant patients despite posaconazole prophylaxis: two cases and review of the literature. Biology of Blood and Marrow Transplantation. 2009;15(8):991-995.
- [130]Spellberg B, Ibrahim AS. Recent advances in the treatment of mucormycosis. Current Infectious Disease Reports. 2010;12(6):423-429.
- [131]Ibrahim AS, Bowman JC, Avanessian V, Brown K, Spellberg B, Edwards Jr JE, et al. Caspofungin inhibits Rhizopus oryzae 1, 3-β-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrobial Agents and Chemotherapy. 2005;49(2):721-727.
- [132]Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, et al. Combination therapy for mucormycosis: why, what, and how?. Clinical Infectious Diseases. 2012;54(suppl_1):S73-S78.
- [133]Martin-Vicente A, Capilla J, Guarro J. Synergistic effect of anidulafungin combined with posaconazole in experimental aspergillosis. Medical Mycology. 2017;55(4):457-460.
- [134]Katragkou A, McCarthy M, Meletiadis J, Petraitis V, Moradi PW, Strauss GE, et al. In vitro combination of isavuconazole with micafungin or amphotericin B deoxycholate against medically important molds. Antimicrobial Agents and Chemotherapy. 2014;58(11):6934-6937.
- [135]Chamilos G, Lewis RE, Kontoyiannis DP. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrobial Agents and Chemotherapy. 2006;50(1):96-103.
- [136]Gebremariam T, Lin L, Liu M, Kontoyiannis DP, French S, Edwards JE, et al. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. The Journal of Clinical Investigation. 2016;126(6):2280-2294.
- [137]Ibrahim AS, Edwards Jr JE, Fu Y, Spellberg B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. Journal of Antimicrobial Chemotherapy. 2006;58(5):1070-1073.
- [138]Liu M, Spellberg B, Phan QT, Fu Y, Fu Y, Lee AS, et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. The Journal of Clinical Investigation. 2010;120(6):1914-1924.
- [139]Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. The Journal of Clinical Investigation. 2007;117(9):2649-2657.
- [140]Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, et al. Zinc and respiratory tract infections: Perspectives for COVID‑19. International Journal of Molecular Medicine. 2020;46(1):17-26.
- [141]Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A, Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358.
- [142]Staats CC, Kmetzsch L, Schrank A, Vainstein MH. Fungal zinc metabolism and its connections to virulence. Frontiers in Cellular and Infection Microbiology. 2013;3:65.
- [143]Leonardelli F, Macedo D, Dudiuk C, Theill L, Cabeza MS, Gamarra S, Garcia-Effron G. In vitro activity of combinations of zinc chelators with amphotericin B and posaconazole against six Mucorales species. Antimicrobial Agents and Chemotherapy. 2019;63(5):e00266-19.
- [144]Abzug MJ, Walsh TJ. Interferon-γ and colony-stimulating factors as adjuvant therapy for refractory fungal infections in children. The Pediatric Infectious Disease Journal. 2004;23(8):769-773.
- [145]Gil-Lamaignere C, Simitsopoulou M, Roilides E, Maloukou A, Winn RM, Walsh TJ. Interferon-γ and granulocyte-macrophage colony-stimulating factor augment the activity of polymorphonuclear leukocytes against medically important zygomycetes. The Journal of Infectious Diseases. 2005;191(7):1180-1187.
- [146]Grimaldi D, Pradier O, Hotchkiss RS, Vincent JL. Nivolumab plus interferon-γ in the treatment of intractable mucormycosis. The Lancet Infectious Diseases. 2017;17(1):18.
- [147]Habib HM, Ibrahim S, Zaim A, Ibrahim WH. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomedicine & Pharmacotherapy. 2021;136:111228.
- [148]Ghannoum M, Long L, Larkin EL, Isham N, Sherif R, Borroto-Esoda K, et al. Evaluation of the antifungal activity of the novel oral glucan synthase inhibitor SCY-078, singly and in combination, for the treatment of invasive aspergillosis. Antimicrobial Agents and Chemotherapy. 2018;62(6):e00244-18.
- [149]Gebremariam T, Alkhazraji S, Alqarihi A, Wiederhold NP, Shaw KJ, Patterson TF, Filler S, Ibrahim A. APX001A Protects Immunosuppressed Mice from Rhizopus delemar Infection. Open Forum Infectious Diseases. 2017;4(suppl_1): S475-S475.
- [150]Ben Yaakov D, Rivkin A, Mircus G, Albert N, Dietl AM, Kovalerchick D, et al. Identification and characterization of haemofungin, a novel antifungal compound that inhibits the final step of haem biosynthesis. Journal of Antimicrobial Chemotherapy. 2016;71(4):946-952.