Association of candidate genes polymorphisms in Iraqi patients with chronic kidney disease
Abstract
In many areas of the globe, chronic kidney disease (CKD) is a widespread health concern defined by the progressive loss of kidney function. The genetic contribution to the development of kidney disease is essential in forecasting risk variables and resolving genetic enigmas. Genetic variations are among the factors that might be linked with renal disease development. This research aims to examine the relationship between five single nucleotide polymorphisms (SNPs): rs1126616, rs35068180, rs1800247, rs4236, and rs2248359 with the risk of developing CKD among Iraqi patients. A study involved 80 subjects, divided into fifty CKD patients and thirty healthy subjects. Genotyping was identified by applying the polymerase chain reaction followed by the restriction fragment length polymorphism (PCR-RFLP) method. Compared to the control group, the recurrences of the genotyping TT (p = 0.01) and their allelic T (p = 0.02) in the rs1126616 were considerably higher in the CKD group. Regarding of rs1800247 CT and TT genotypes and (T) allele exhibited a substantial excess in the CKD patients (p = 0.01, 0.0229, < 0.03, respectively). For rs4236, the TT genotype (p = 0.01) and T (p = 0.02) allele were significantly increased in the CKD patients. The recurrences of the genotyping TT (p = 0.02) and their allelic T (p = 0.01) in the rs2248359 were considerably greater in the CKD group. Furthermore, the genotypes and alleles of rs35068180 showed no significant variations among CKD patients and healthy subjects. The results demonstrated that four genetic polymorphisms are probably biomarkers or effective factors linked to CKD patients. Also, specific genetic polymorphisms might lower or raise a patient’s renal disease risk. Differences in genetic and allelic patterns can significantly impact how a disease is treated and what medicines are used.
INTRODUCTION
Chronic kidney disease (CKD) is a prevalent health problem in many states that involves the gradual cessation of kidney function as waste and fluid accumulate in the body and are not excreted in the urine [1]. In addition to advancing age, genetic and environmental factors, diabetes mellitus, high blood pressure, and cardiovascular disease may raise the risk of CKD, which can develop into end-stage renal failure, which can be fatal [2, 3].
Genetic influences can be involved in determining the course of renal disease and the possibility of its development through advanced modern techniques that review the effect of genetic change on the phenotype through the course of the natural disease, susceptibility to infection, and reactions to preventive treatments [4].
Several studies utilizing advanced technologies have contributed to the assessment of genetic risk factors and the identification of the roles of genetic variants associated with chronic kidney failure through the modification of the coding of some genes containing single-nucleotide polymorphisms and their crucial roles in predicting the risk of CKD [5-7].
There is much evidence referring to the genetic contribution to kidney disease development. Some researchers have established a link between renal failure and the modification of protein expression and genetic differences present in numerous human genes. These genes are involved in predicting risk factors and elucidating genetic conditions related to the diseases including osteopontin (OPN), matrix metallopeptidase-3 (MMP-3), osteocalcin (OCN), matrix gamma-carboxyglutamic acid protein gene (MGP), and 24-hydroxy [8-12].
OPN is a glycoprotein found in many secretions, cells, and organs that plays a role in bone resorption and serves as a controller of immunological reactions. It also protects against kidney disease, oxidative stress, and nephrolithiasis [13]. Furthermore, the OPN SNP rs1126616 is linked to kidney disease and has increased chronic kidney disease and kidney dysfunction [14]. Moreover, a relationship was found between the recurrences of (SNPs) at the OPN gene and the nephritic complications, which may be useful as biomarkers for rejecting kidney transplants [8]. On the other hand, the MMP-3 gene or stromelysin-1 are anti-fibrotic factors involved in many physiological actions that are expressed in many tissues of the body and linked with the regulation of renal diseases that can be seen in CKD and inflamed renal tissues [15]. According to mounting data, MMPs appear to have a wide range of roles in the development of new blood vessels, inflammation, proliferation, and apoptosis, which are typically caused by an immune response, as well as a variety of kidney disorders, including renal damage, CKD, and glomerulonephritis [15-17].
However, nephropathy in diabetics has been associated with the rs35068180 SNPs in the gene that codes for the MMP-3 gene [18]. Its conjunction with the MMP-1 gene is highly related to kidney fibrosis and end-stage renal failure. The MMP-3 gene suppression can ameliorate fibrosis and damage in the renal tissues [19].
The OCN gene is a protein substantially more prevalent in osteocytes, dependent on potassium supplements, and associated with anticancer activity [20]. It is also involved in the structure of bone matrix calcification pathways.
The OCN gene may decrease intravenous calcination by influencing the vitamin D gene activity in humans. Indicating that the beneficial role of calciferol on membrane thin layer cell damage is intermediated by a lowering in the expression of (OCN) protein in calcination pathways [21]. Vitamin D deficiency is common in most patients with renal disease [22].
The harmful interaction between the brittle skeleton and the rigidity of the arterial wall results from osteoporosis and vascular calcification developing in chronic renal failure. So, bone-related OCN proteins were given importance, and a relationship investigation between SNPs-rs1800247 of OCN coding gene, bone gamma-carboxyglutamate protein (BGLAP), and chronic renal failure development was carried out, which serve as regulators or activators and mediate between the bone and vasculature [23, 24].
Otherwise, the MMP-3 gene, called MGP, can be found in many organs and is a well-known suppressor of calcination in the vasculature. It is abundant in the calcinated atheroma plaques in people. The potassium deficiency, linked to impaired MGP activity in the vessels, is a significant predictor of vascular calcination and osteoporosis in renal failure patients [25]. Moreover, the polymorphisms of the MGP gene have been identified in patients with CKD to estimate the affinity between genotype and disease development by rs4236 SNPs related to the MGP gene that may be a biomarker for the development in end-stage patients of kidney failure [11].
The decreased vitamin D and phosphate levels in patients with kidney failure are associated with an increased expression of the CYP24A1 genes, which are related to the cytochrome P450 enzyme that stimulates the processing of 25-hydroxy vitamin D3 and 1,25-dihydroxy vitamin D3 to form vitamin D-24 hydroxylase [12, 26]. In the same context, rs2248359 SNPs are located in the CYP24A1 gene that codes the 24-hydroxylase enzyme, contributing to the regulation of renal damage exacerbated [27].
However, declining vitamin D can aggravate calcium and phosphate balance, leading to kidney-bone abnormalities in patients with CKD and other renal dysfunction consequences. These outcomes support a greater emphasis on suppressing CYP24A1 action to treat vitamin D insufficiency in patients with CKD [28]. For other applications, the principal objective of this research is to identify the linkage between gene polymorphisms and the risk of developing CKD, the potential for solid significance between the polymorphism of these genes and the development of CKD, and the possibility of using genes as biological biomarkers in detecting CKD.
MATERIALS AND METHODS
Subjects of the study
Samples were collected from volunteer subjects at hospitals and medical clinics in Anbar Governorate, Iraq. Doctors diagnosed the samples of patients with CKD according to patient reports. The ethical clearance was done according to the Iraqi Ministry of Health and Environment (15.02.2021-15.02.2022).
This study was conducted in the Molecular Genetics Laboratory, College of Science, University of Anbar. The study involved 80 subjects aged (25–70) years, divided into 50 patients with CKD (26 and 24) for men and women, respectively. It excluded the subjects with a history of acute kidney failure, liver failure, malignancy, and infectious disease. The control group involved 30 healthy subjects (19 and 11), men and women, respectively, with the absence of health disorders or any history of CKD.
Sampling and DNA extraction
Under sterile conditions, 5 ml of whole blood was drawn from each participant and placed in ethylenediaminetetraacetic acid (EDTA) tubes. Double-stranded nucleic acid (DNA) was extracted through a genomic DNA purification kit (Promega, USA), depending on the manufacturer’s procedures.
Genotyping and polymorphisms
The running investigation examined five polymorphisms: rs1126616 at the OPN gene, rs35068180 at the MMP-3 gene, rs1800247 at the OCN gene, rs4236 at the MGP gene, and rs2248359 at the CYP24A1 gene were chosen. Genotyping was identified by applying the polymerase chain reaction followed by the restriction fragment length polymorphism (PCR-RFLP) method.
In addition, the five studied polymorphisms (rs1126616, rs35068180, rs1800247, and rs2248359) were evaluated by applying specific methods for each gene, which included enzymatic amplification procedures, thermocycling conditions, primer sequences, and restriction enzymes used for each gene, as shown in Table 1. These methods were based on genetic analysis methods proposed by previous research.
From Table 1, a polymerase chain reaction PCR (Thermo-Fisher-Scientific, USA) device was used for all amplification reactions. Depending on the manufacturer’s steps, five restriction enzymes specific to each gene were used to digest the products when PCR products were generated. The size of PCR-amplicons of single nucleotide polymorphisms (SNPs) rs1126616 (250 bp), rs35068180 (129 bp), rs1800247 (253 bp), rs4236 (173 bp), and rs2248359 (326 bp) was digested by AluI, PsyI, HindIII, Eco477, and SacII enzyme, respectively.
The PCR was used to obtain PCR and enzyme products, and electrophoresis with 2 % agarose gel was used to examine and isolate the PCR and enzyme products.
Table 1. Screening of polymorphisms genotyping with products of PCR and restriction enzymes.
Statistical analysis
SPSS software (modeler-18.0, United States) was used to analyze the data statistically. The associations between genetic polymorphisms and CKD were evaluated by comparing allele and genotype frequencies in patients and healthy subjects by (Chi-squared and Fisher’s Exact) tests. Evaluating the odds ratio (OR) with a 95% confidence interval (CI) and a probability (p) value that is below five percent (p = 0.05) is taken into account to be statistically meaningful.
RESULTS
The OPN gene and CKD
Table 2 represents the frequencies of the rs1126616 genotyping and its alleles among the CKD patients and control (healthy subjects) groups. The genotype frequency for the rs1126616 polymorphism, CC, CT, and TT, is (8%, 28%, and 64%) in the CKD group (76.66%, 20%, and 3.333%) in the control group, respectively.
Recurrence of the (C and T) alleles was seen in (22% and 78%) of CKD patients, compared to (86.66% and 13.33%) of controls. The recurrence of the rs1126616 TT genotype was expressively excess in kidney patients at 64% when compared to the healthy subjects at 3.33% (p = 0.01, OR = 0.01940, 95% CI = 0.001841-0.1329).
Also, the recurrence of the (T) allele was significantly higher in the CKD group (78% compared to the healthy subjects 13.33 % (p = 0.02, OR = 0.04339, 95% CI = 0.01874 to 0.1036) as shown in Figure 1.
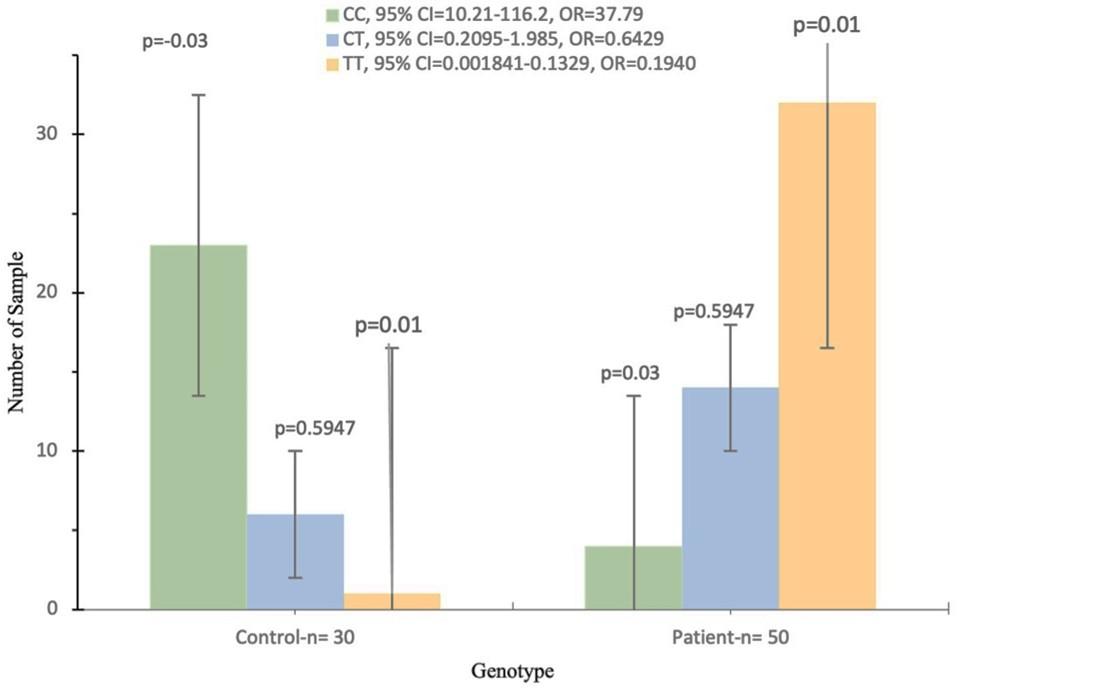
Table 2. In CKD patients, genotypic and allelic frequencies (%) of OPN gene rs1126616 polymorphism.
The MMP-3 gene and CKD
Table 3 shows how the genotypes and alleles of the MMP-3 gene rs35068180 polymorphism were spread out among people with CKD and people who were controlled. CKD group, the frequency of the rs35068180 polymorphism genotypes consists of (5A/5A = 16%, 5A/6A = 64%, and 6A/6A = 20%), while in healthy subjects it is (5A/5A = 33.33%, 5A/6A = 46.67%, and 6A/6A = 20%). However, the frequency of the rs35068180 alleles consists of (5A = 48% and 6A = 52%) in CKD patients, whereas the allelic in healthy subjects was (5A = 56.67% and 6A = 43.33%).
Furthermore, the proportions of rs35068180 polymorphism genotypes and alleles among CKD patients and healthy subjects showed insignificant variations that were not statistically significant, despite the frequency of the rs35068180 5A/6A genotype being high in the CKD patients versus the healthy subjects (64% vs 46.67%). Still, the variation was also not statistically significant.
Table 3. In CKD patients, the genotypic and allelic frequencies (%) of MMP-3 gene rs35068180 polymorphism.
The relationship between CKD and the OCN gene
Results of CKD patients and healthy subjects on the genotyping and allelic recurrence of the OCN-rs1800247 polymorphism are described in Table 4. Regarding rs1800247 polymorphism, the frequency of the genotypes CC, CT, and TT consists of (4%, 68%, and 28%) in the CKD group and (80%, 13.33%, and 6.66%) in the control group, respectively.
While the rs1800247 allele frequencies (C and T) were seen in (38% and 62%) of CKD patients and (86.66% and 13.3%) of healthy individuals, respectively. The current data indicated that the frequency of the rs1800247 polymorphism genotypes (CT and TT) showed a significant increase in the CKD group (68% and 28%) than those of the control group (13.33% and 6.66%) (p = 0.001, 0.0229, OR = 0.07240, 0.1837, 95% CI = 0.02492-0.2447, 0.03953 to 0.8360, respectively).
Likewise, the allelic frequency (C) showed a significant increase in the CKD patients (62%) compared to the control group (13.33%) (p = 0.01, OR = 0.09429, 95% CI = 0.04306-0.2241) as shown in Figure 2.
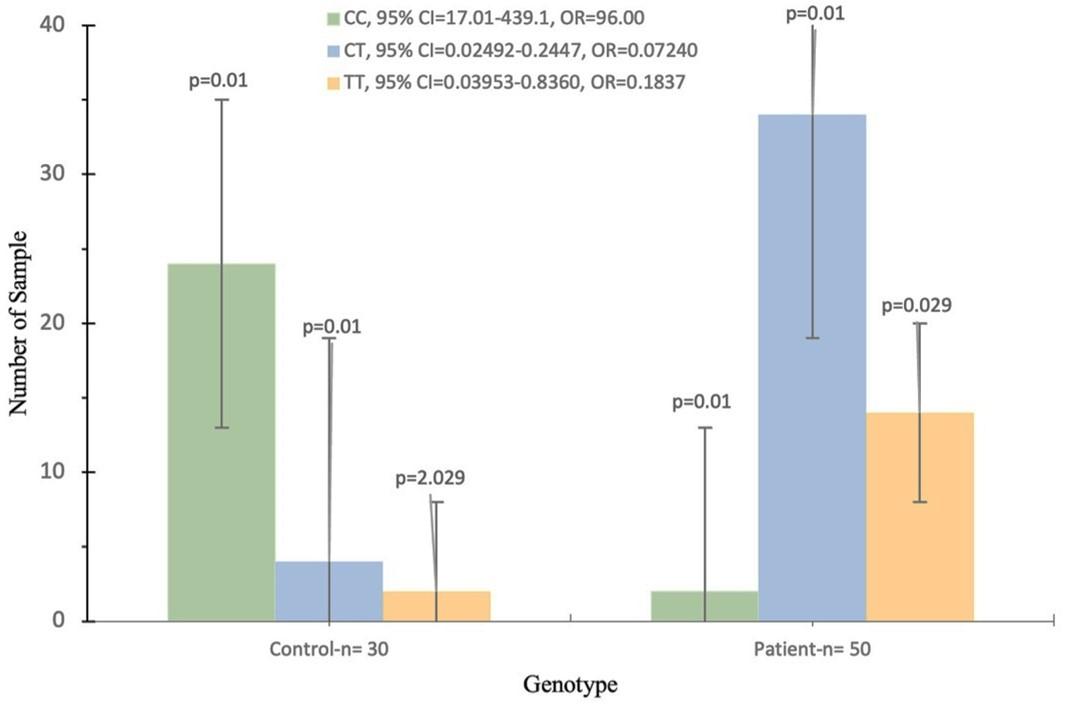
Table 4. In CKD patients, the genotypic and allelic frequencies (%) of OCN gene rs1800247 polymorphism.
The MGP gene and CKD
Table 5 shows the genotyping and allelic recurrences of the MGP gene rs4236 polymorphism in people with CKD and those in control. For rs4236, the (CC, CT, and TT) genotypes make up (4%, 12%, and 78%) of the CKD patients and (83.33%, 10%, and 6.7%) of the healthy control group, respectively. The frequency of the rs4236 allele (C and T) was (13% and 87%) in CKD patients and was (88.33% and 11.67%) in control, respectively.
Moreover, an elevated rate of recurrence of the genotype (TT) was detected in the CKD patients at 78% when compared with the healthy control group at 6.67%; thus, this genotype of rs4236 polymorphism might be associated with an increased risk of CKD (p = 0.01, OR = 0.02015, 95% CI, 0.004498-0.09359).
However, the rs4236-T allele was remarkably excess in the CKD patients 87% compared to the healthy subjects 13%, so this allele may be associated with an increased risk of CKD (p = 0.02, OR = 0.0197, 95% CI, 0.008311-0.05140) as shown in Figure 3.
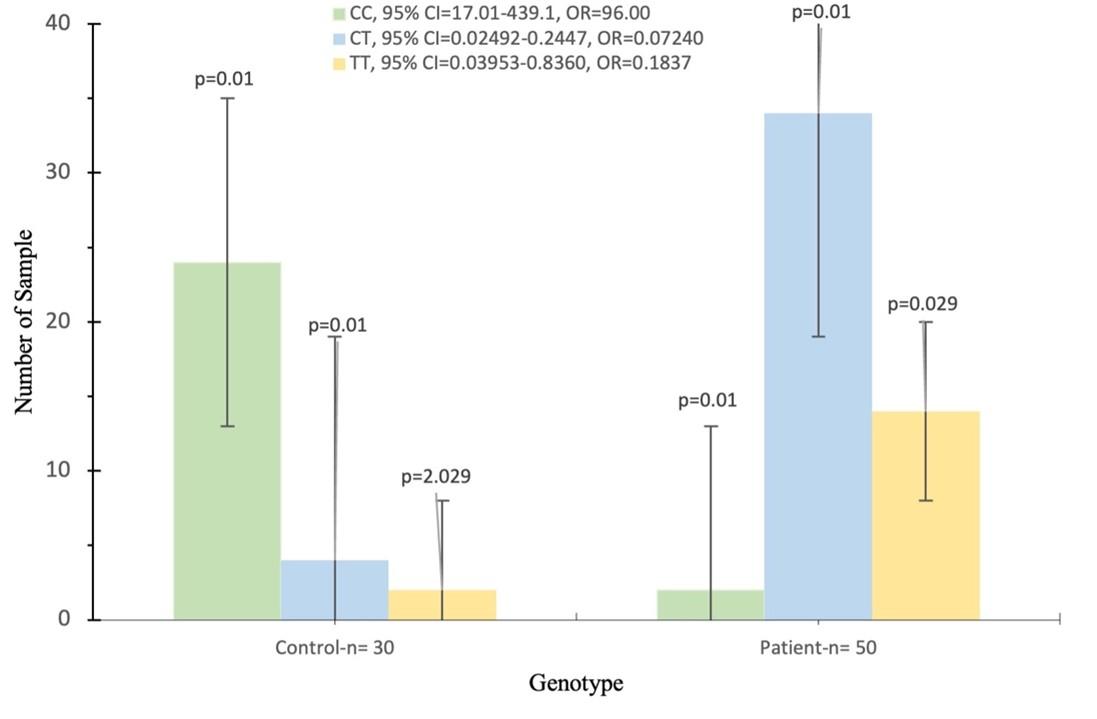
Table 5. The percentages of CKD patients with an MGP gene rs4236 polymorphism.
The CYP24A1 gene and CKD
Table 6 indicates the frequencies of the CYP24A1 gene rs2248359 genotypes and their alleles between the CKD and control groups. The genotyping frequencies for the CYP24A1 gene rs2248359 polymorphism, the (CC, CT, and TT) consist of (6, 22, and 72) % in the patients with CKD group, and (86.66, 13.33, and 0) % in the control group, respectively. The frequency of the rs2248359 (C and T) alleles was found in (17 and 83) % of the CKD patients, respectively, while it was (93.33 and 6.66) % of the control group, respectively. The frequency of the rs2248359 (TT) genotype was significantly elevated in the CKD group (72%) compared to the control group (0%) (p = 0.02, OR = 0.0065, 95% CI = 0.01874-0.1036). The frequency of the (T) allele was significantly higher in the CKD group (83%) compared to the control group (6.66%) (p = 0.01, OR = 0.01463, 95% CI = 0.005342-0.04586) as shown in Figure 4.
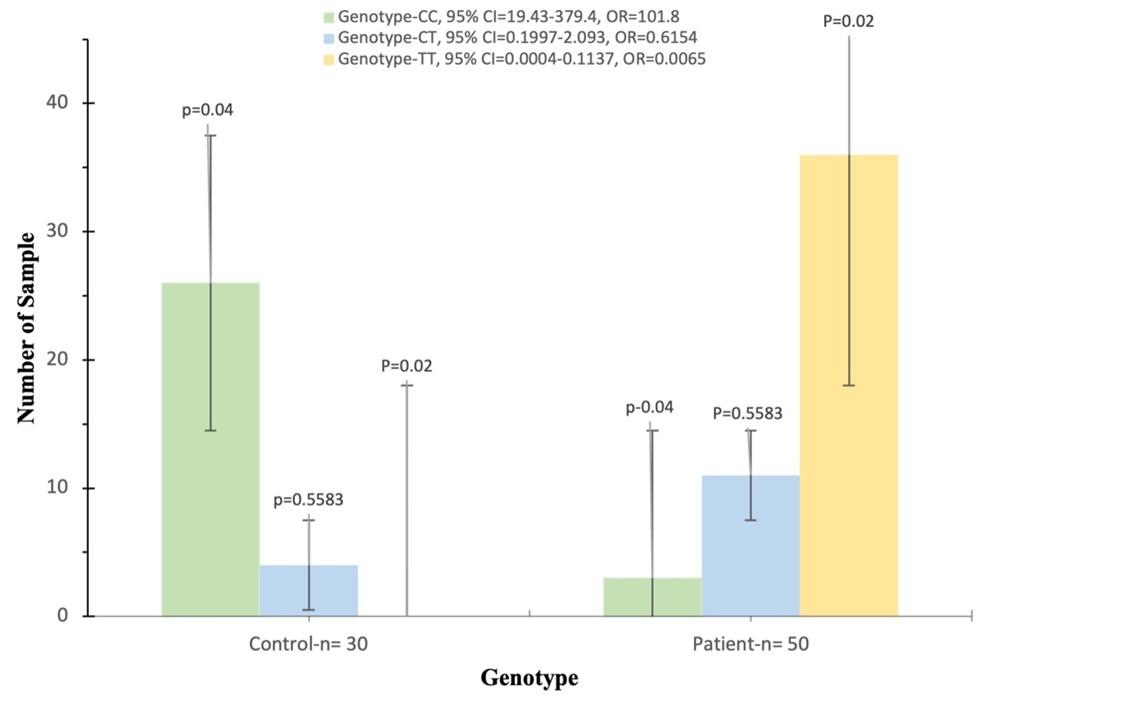
Table 6. Genotypic and allelic frequencies (%) of CYP24A1 gene rs2248359 polymorphism in CKD patients.
DISCUSSION
SNPs linked to or located at genes have been extensively studied in many human disorders, including kidney, heart, pancreatic, infectious, and malignant diseases. A category of Iraq sufferers with chronic renal disease for five genes that involve OPN gene, MMP-3 gene, OCN gene, MGP gene, and CYP24A1 gene, respectively for genotype were estimated. During the five polymorphisms analysis of the CKD patients compared to the control group, our results showed an association with four SNPs, including rs1126616, rs1800247, rs4236, and rs2248359, via observing significant elevations in their genetic and allelic frequencies. At the same time, we did not find any association with the SNPs rs35068180, and their genetic and allelic frequencies showed no significant variations.
Many complications and physiological disorders are associated with CKD patients, which can lead to human death. However, several studies indicate some disorders may be associated with kidney failure but are clinically not completely clear [34]. Among those problems and complications, atherosclerosis, heart and blood vessel disease cases are the complications introduced by patients with renal failure [35, 36].
Numerous studies have shown the significant impact of the OPN gene polymorphism rs1126616 in the progression of malignancies and the likelihood of cancer in people [37]. The polymorphism of the OPN gene rs1126616 plays a significant role in tumorigenesis. It is linked to an elevated risk of colon cancer, and the prevalence of polymorphisms OPN gene is affected by demographic characteristics [38].
The outcomes showed an association in the genetic and allelic frequencies of the rs1126616 polymorphism in CKD, as there was a significant increase in the genetic (TT) and allelic (T) frequencies among kidney patients compared to the control group. These results corroborate a study conducted on lupus nephropathy patients, which revealed an increase in genotype frequencies (TT) of the OPN gene in lupus nephropathy patients compared to healthy subjects [30]. Also, previous research supports the findings, which describe that the rs1126616 SNPs are linked to cases of renal failure and cardiac disease. In addition, elevated patterns of the OPN gene have been detected in patients with severe renal complications [8].
SNP rs35068180 is essential for contributing to the pathological and physiological roles of the kidneys by inducing ameliorative effects in injured kidney tissues and other renal functions [39]. MMP-3 gene encodes an enzyme that breaks down proteins and peptides outside of a cell in a regulated manner. Some matrices patterns have been found in hepatic tissue [40]. The genotype patterns of the MMPs gene regulate the development and growth of the endothelium cells and involuntary non-striated muscle, influencing the progression of the vascular endothelium, blood-vessel reorder, and maybe inducing high blood pressure [41-42].
The effect of the MMP-3 gene was indistinct at the biological level in current findings due to the low genetic and allelic frequencies of SNPs rs35068180 in Iraqi CKD patients. There was a low number of genetic (TT) and allelic (T) frequencies in kidney disease patients. Nevertheless, this might be a marker or a protective signal, and previous researchers’ remarks were split about the protective functions of these genes play in kidney disease patients
Often, the genetic analyses do not correlate with one another on the function of rs35068180 polymorphism as indicators in the evolution of high blood pressure and may change in various communities according to the demographic origin, participant number, and methodology in the study [43]. In another study, associations between three patterns of the MMP-3 genes and frequent abortion in Iranian women were examined. Their results found that the rs35068180 genotype variation was most common in patients with miscarriages, while no association was found with polymorphisms in other patterns of MMPs genes [44].
The OCN gene rs1800247 polymorphism is prominent in the coding position of the OCN gene that has been linked to blood pressure and bone injuries in numerous studies [45-47]. In contrast, previous studies did not find prominent roles or sufficient information for the rs1800247 polymorphism at the renal level. An association between rs1800247 and kidney patients has been found. It is unclear how or why this gene affects the start and progression of renal failure, but it could have a role because it is in the regulatory region, causes a change in the nucleotides, and may control gene expression. The findings showed an elevated frequency of the (TT) genotype and (T) allele of the MGP gene rs4236 polymorphism in the patients with CKD compared to the control group.
Therefore, these genotypic and allelic variants of rs4236 might be related to an increased risk of CKD. Moreover, a Ukrainian study reported the presence of the (MGP) rs4236 polymorphism in female and male CKD patients with complications of myocardial infarction, low blood flow, and blood vessel diseases. Their genetic analyses showed a significantly increased frequency of the rs4236 polymorphism (TT) genotype and the (T) allele in the CKD patients compared to the healthy group.
They stated that the rs4236 genetic polymorphism in patients who show negative symptoms and poor performance of the heart and blood vessels associated with renal failure could be a biomarker of these diseases and their dangerous complications [32]. Furthermore, the MGP gene can be organized by calcination in external cell matrices. It is overexpressed in many tissues, including the heart, blood vessels, renal, skeleton, and pulmonary tissues [11].
The MGP gene was highly expressed in the epithelium cells of kidney tissues and people with a fatty deposit on a blood vessel [48]. A Chinese study was conducted on men and women patients with renal calculi to detect the genotypes of the polymorphism MGP gene rs4236. They found that rs4236 strongly correlates with renal calculi patients and may be an indicator to predict the risk of developing renal calculi. The efficiency of the MGP gene is likely linked to the inner membrane of blood vessel autonomic myocytes [49].
However, the genotyping of MGP gene rs4236 polymorphism and variations in the allele can cause an alteration in the amino acid sequence and translation process of the protein, which may influence the development of non-soluble calcium synthesis in kidney patients [50]. The links between the CYP24A1 gene rs2248359 polymorphism and the danger of CKD among Iraqi individuals have been investigated for genetic analysis.
A comparison of rs2248359 genotype frequencies in the CKD patients and control groups showed that the (TT) genotypes and (T) allele portions were linked to an elevated risk of CKD. Our findings revealed that CKD patients with high (TT) genotypes and (T) alleles had a higher chance of developing CKD. The diagnostic variables of the rs2248359 polymorphism of these results could be helpful in risk evaluation or as potential diagnostic markers for CKD.
The CYP24A1 genotype has been linked to renal disease and calcium levels in genomic investigations because of its critical role in the metabolic processes of vitamin D [13]. The relationship between rs2248359 variants and the metabolic pathway of vitamin D insufficiency has been identified in many communities [51]. Within that investigation, we successfully amplified the CYP24A1 gene expressing hydroxylase in the biological process of vitamin D. We discovered a connection between rs2248359 SNPs and CKD patients, and our outcomes indicated that CYP24A1 gene variants might raise the risk of CKD [50, 51].
As very few studies have suggested rs2248359 polymorphisms with CKD, these genetic variations have been widely researched in other disorders, particularly heart disease, metabolic disorders, autoimmunity, endocrine disorders, and various malignancies [52-54]. Furthermore, [12, 28, 52-60] showed that CYP24A1 gene variants and their alleles are associated with people with renal disorders such as increased serum and urine calcium levels, precipitation of salts and minerals in the renal tissues, and renal sacks.
As a result, more research into the role of polymorphisms in CKD patients must be promoted to fully comprehend the mechanism underlying the reported link to renal illness. For individuals still in the early stages of CKD, a better understanding of the effect of these polymorphisms might be beneficial in developing new therapies for them. Additional analyses of the genetic variations in other population categories will allow us to recognize better the linked processes of the CKD and which support or contradict our results. Moreover, the findings of this research potentially give a clear picture of gene variants in CKD patients and identify analytical, clinical, and preventive indicators that contribute to the management, early diagnosis, and treatments of many diseases.
CONCLUSION
Genetic analyses in this study propose that SNPs are likely to be a biomarker or effective participator linked to CKD in the Iraqi populations, as increases in the genetic variation and allelic frequencies were detected in four SNPs included rs1126616, rs1800247, rs4236, and rs2248359. Moreover, no express variations in the genotyping and allelic recurrences of the SNPs-rs35068180 were detected between CKD patients and control people. Furthermore, appropriate genetic polymorphisms might alleviate or increase the risk of renal disease in patients, and the variations in genetic and allelic patterns can play essential roles in disease management and medication. However, it is suggested that more genetic study designs be conducted to demonstrate the similarities or differences between these findings via selecting large and different social models to investigate the role of polymorphisms in genetic associations.
ACKNOWLEDGEMENTS
None.
AUTHORS CONTRIBUTION
AJH, TFM; methodology, MNA, and LHS; designed and performed the experiments, MNA, LHS, and MR; analyzed and interpreted the data. MNA, and MR; prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. The lancet. 2017; 389(10075): 1238-1252.
- [2]Eriksen BO, Stefansson VT, Jenssen TG, Mathisen UD, Schei J, Solbu MD, Wilsgaard T, Melsom T. Elevated blood pressure is not associated with accelerated glomerular filtration rate decline in the general non-diabetic middle-aged population. Kidney international. 2016; 90(2): 404-10.
- [3]Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, Zhang X, Roy JA, Lustigova E, Nessel L, Ford V. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA cardiology. 2017; 2(6): 635-43.
- [4]Qiu C, Huang S, Park J, Park Y, Ko YA, Seasock MJ, Bryer JS, Xu XX, Song WC, Palmer M, Hill J. Renal compartment–specific genetic variation analyses identify new pathways in chronic kidney disease. Nature medicine. 2018; 24(11): 1721-1731.
- [5]Geddes RF, Jepson RE, Forcada Y, Elliott J, Syme HM. Associations between single nucleotide polymorphisms in the calcium sensing receptor and chronic kidney disease-mineral and bone disorder in cats. The Veterinary Journal. 2018; 235: 34-41.
- [6]Albert C, Kube J, Albert A, Schanze D, Zenker M, Mertens PR. Cubilin single nucleotide polymorphism variants are associated with macroangiopathy while a matrix metalloproteinase-9 single nucleotide polymorphism flip-flop may indicate susceptibility of diabetic nephropathy in type-2 diabetic patients. Nephron. 2019; 141(3): 156-65.
- [7]Corredor Z, Rodríguez-Ribera L, Velázquez A, Hernández A, Catalano C, Hemminki K, Coll E, Silva I, Diaz JM, Ballarin J, Vallés Prats M. Genetic variants associated with chronic kidney disease in a Spanish population. Scientific reports. 2020; 10(1): 1-1.
- [8]Cambray S, Galimudi RK, Bozic M, Bermúdez-López M, Rodríguez I, Valdivielso JM. The rs1126616 single nucleotide polymorphism of the osteopontin gene is independently associated with cardiovascular events in a chronic kidney disease cohort. Journal of clinical medicine. 2019; 8(5): 592.
- [9]Robinson C, Tsang L, Solomon A, Woodiwiss AJ, Gunter S, Millen AM, Norton GR, Fernandez-Lopez MJ, Hollan I, Dessein PH. Omentin concentrations are independently associated with those of matrix metalloproteinase-3 in patients with mild but not severe rheumatoid arthritis. Rheumatology international. 2017; 37(1): 3-11.
- [10]Marchelek-Mysliwiec M, Wisniewska M, Nowosiad-Magda M, Safranow K, Kwiatkowska E, Banach B, Dołegowska B, Dołegowska K, Stepniewska J, Domanski L, Pawlik A. Association between plasma concentration of klotho protein, osteocalcin, leptin, adiponectin, and bone mineral density in patients with chronic kidney disease. Hormone and Metabolic Research. 2018; 50(11): 816-821.
- [11]Jaminon AM, Dai L, Qureshi AR, Evenepoel P, Ripsweden J, Söderberg M, Witasp A, Olauson H, Schurgers LJ, Stenvinkel P. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Scientific reports. 2020; 10(1): 1-9.
- [12]Hanna C, Potretzke TA, Cogal AG, Mkhaimer YG, Tebben PJ, Torres VE, Lieske JC, Harris PC, Sas DJ, Milliner DS, Chebib FT. High prevalence of kidney cysts in patients with CYP24A1 deficiency. Kidney International Reports. 2021; 6(7): 1895-903.
- [13]Si J, Wang C, Zhang D, Wang B, Hou W, Zhou Y. Osteopontin in bone metabolism and bone diseases. Medical science monitor: international medical journal of experimental and clinical research. 2020; 26: e919159-1.
- [14]Vianello E, Kalousová M, Dozio E, Tacchini L, Zima T, Corsi Romanelli MM. Osteopontin: The molecular bridge between fat and cardiac–renal disorders. International Journal of Molecular Sciences. 2020; 21(15): 5568.
- [15]Yazgan B, Avcı F, Memi G, Tastekin E. Inflammatory response and matrix metalloproteinases in chronic kidney failure: Modulation by adropin and spexin. Experimental Biology and Medicine. 2021; 246(17): 1917-1927.
- [16]Amin M, Pushpakumar S, Muradashvili N, Kundu S, Tyagi SC, Sen U. Regulation and involvement of matrix metalloproteinases in vascular diseases. Frontiers in bioscience (Landmark edition). 2016; 1(21): 89.
- [17]Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuna JM, Perez-Romero BA, Guerrero-Rodriguez JF, Martinez-Avila N, Martinez-Fierro ML. The roles of matrix metalloproteinases and their inhibitors in human diseases. International journal of molecular sciences. 2020; 21(24): 9739.
- [18]Srilatha Reddy G, Surekha Rani H. Matrix metalloproteases: Potential role in type 2 diabetic nephropathy. Pathophysiological Aspects of Proteases. 2017; (pp. 605-616).
- [19]Ay A, Alkanli N, Cevik G. Investigation of the relationship between MMP-1 (− 1607 1G/2G), MMP-3 (− 1171 5A/6A) gene variations and development of bladder cancer. Molecular Biology Reports. 2021; 48(12): 7689-7695.
- [20]Lee KH, Lee KJ, Kim TY, Hutomo F, Sun HJ, Cheon GJ, Park SI, Cho SW, Im SA. Circulating osteocalcin‐positive cells as a novel diagnostic biomarker for bone metastasis in breast cancer patients. Journal of Bone and Mineral Research. 2020; 35(10): 1838-1849.
- [21]Miyamoto T, Oguma Y, Sato Y, Kobayashi T, Ito E, Tani M, Miyamoto K, Nishiwaki Y, Ishida H, Otani T, Matsumoto H. Elevated creatine kinase and lactic acid dehydrogenase and decreased osteocalcin and uncarboxylated osteocalcin are associated with bone stress injuries in young female athletes. Scientific reports. 2018; 8(1): 1-10.
- [22]Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, Kaczmarska M, Stefańczyk L, Vermeer C, Maresz K, Nowicki M. Plasma desphospho-uncarboxylated matrix Gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney and Blood Pressure Research. 2016; 41(3): 231-9.
- [23]Zhang XY, He JW, Fu WZ, Liu YJ, Zhang ZL. Associations of serum osteocalcin and polymorphisms of the osteocalcin gene with bone mineral density in postmenopausal and elderly Chinese women. Lifestyle Genomics. 2016; 9(5-6): 231-242.
- [24]Valls J, Cambray S, Pérez-Guallar C, Bozic M, Bermúdez-López M, Fernández E, Betriu À, Rodríguez I, Valdivielso JM. Association of candidate gene polymorphisms with chronic kidney disease: results of a case-control analysis in the nefrona cohort. Frontiers in genetics. 2019; 10: 118.
- [25]Barrett H, O’Keeffe M, Kavanagh E, Walsh M, O’Connor EM. Is matrix Gla protein associated with vascular calcification? A systematic review. Nutrients. 2018; 10(4): 415.
- [26]Meyer MB, Pike JW. Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression. The Journal of steroid biochemistry and molecular biology. 2020; 196:105500.
- [27]Solache-Berrocal G, Rolle-Sóñora V, Martín-Fernández N, Cambray S, Valdivielso JM, Rodríguez I. CYP24A1 and KL polymorphisms are associated with the extent of vascular calcification but do not improve prediction of cardiovascular events. Nephrology Dialysis Transplantation. 2021; 36(11): 2076-2083.
- [28]Allegra S, Cusato J, De Francia S, Arduino A, Longo F, Pirro E, Massano D, De Nicolò A, Piga A, D’avolio A. Role of CYP24A1, VDR and GC gene polymorphisms on deferasirox pharmacokinetics and clinical outcomes. The Pharmacogenomics Journal. 2018; 18(3): 506-15.
- [29]Salimi S, Noora M, Nabizadeh S, Rezaei M, Shahraki H, Milad MK, Naghavi A, Farajian-Mashhadi F, Zakeri Z, Sandoughi M. Association of the osteopontin rs1126616 polymorphism and a higher serum osteopontin level with lupus nephritis. Biomedical Reports. 2016; 4(3): 355-360.
- [30]Ibrahim FA, Elfeky SE, Haroun M, Ahmed MA, Elnaggar M, Ismail NA, Abd El Moneim NA. Association of matrix metalloproteinases 3 and 9 single nucleotide polymorphisms with breast cancer risk: A case‑control study. Molecular and Clinical Oncology. 2020; 13(1): 54-62.
- [31]Chumachenko YD, Harbuzova VY, Ataman AV. Association study between BGLAP gene hindIII polymorphism and type 2 diabetes mellitus development in ukrainian population. Journal of Diabetes Research. 2019; 2019.
- [32]Garbuzova VY, Gurianova VL, Stroy DA, Dosenko VE, Parkhomenko AN, Ataman AV. Association of matrix Gla protein gene allelic polymorphisms (G− 7→ A, T− 138→ C and Thr83→ Ala) with acute coronary syndrome in the Ukrainian population. Experimental & Clinical Cardiology. 2012; 17(1): 30.
- [33]Skalli A, Tazi-Ahnini R, Mpandzou GA, Tibar H, Bouslam N, Benomar A, Hajjout K, Yahyaoui M, El Fahime E, Bouhouche A. Association study of two polymorphisms in vitamin D pathway with multiple sclerosis in the Moroccan population. World Journal of Neuroscience. 2017; 7(4): 363-375.
- [34]Chironda G, Bhengu B. Contributing factors to non-adherence among chronic kidney disease (CKD) patients: a systematic review of literature. Medical & Clinical Reviews. 2016; 2(4): 29.
- [35]Schlieper G, Schurgers L, Brandenburg V, Reutelingsperger C, Floege J. Vascular calcification in chronic kidney disease: an update. Nephrology Dialysis Transplantation. 2016; 31(1): 31-39.
- [36]Valdivielso JM, Rodríguez-Puyol D, Pascual J, Barrios C, Bermúdez-López M, Sánchez-Niño MD, Pérez-Fernández M, Ortiz A. Atherosclerosis in chronic kidney disease: more, less, or just different. Arteriosclerosis, thrombosis, and vascular biology. 2019; 39(10):1938-1966.
- [37]Kamal A, Darwish RK, Saad S, Salama M, El-Baradie TS, Mahmoud HG, Elshiwy Y. Association of osteopontin gene polymorphisms with colorectal cancer. Cancer investigation. 2017; 35(2): 71-7.
- [38]Briones-Orta MA, Avendaño-Vázquez SE, Aparicio-Bautista DI, Coombes JD, Weber GF, Syn WK. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2017; 1868(1): 93-108.
- [39]Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. American journal of physiology-renal physiology. 2012; 302(11): F1351-61.
- [40]Shin HP, Lee JI, Jung JH, Yim SV, Kim HJ, Cha JM, Park JB, Joo KR, Hwang JS, Jang BK. Matrix metalloproteinase (MMP)-3 polymorphism in patients with HBV related chronic liver disease. Digestive diseases and sciences. 2008; 53(3): 823-829.
- [41]Rouis M. Matrix metalloproteinases: a potential therapeutic target in atherosclerosis. Current Drug Targets-Cardiovascular & Hematological Disorders. 2005; 5(6): 541-548.
- [42]Huang XY, Han LY, Huang XD, Guan CH, Mao XL, Ye ZS. Impact of 5A/6A polymorphism of matrix metalloproteinase-3 on recurrent atherosclerotic ischemic stroke in Chinese. International Journal of Neuroscience. 2016; 126(10): 936-941.
- [43]Munshi A, Rajeshwar K, Kaul S, Al‐Hazzani A, Alshatwi AA, Shafi G, Balakrishna N, Jyothy A. Association of tumor necrosis factor‐α and matrix metalloproteinase‐3 gene variants with stroke. European Journal of Neurology. 2011; 18(8): 1053-1059.
- [44]Behforouz A, Dastgheib SA, Abbasi H, Karimi-Zarchi M, Javaheri A, Hadadan A, Tabatabaei RS, Meibodi B, Neamatzadeh H. Association of MMP-2, MMP-3, and MMP-9 polymorphisms with susceptibility to recurrent pregnancy loss. Fetal and Pediatric Pathology. 2021; 40(5): 378-86.
- [45]Ling Y, Gao X, Lin H, Ma H, Pan B, Gao J. A common polymorphism rs1800247 in osteocalcin gene is associated with hypertension and diastolic blood pressure levels: The Shanghai Changfeng study. Journal of Human Hypertension. 2016; 30(11): 679-684.
- [46]Boyacioglu O, Orenay-Boyacioglu S, Yildirim H, Korkmaz M. Boron intake, osteocalcin polymorphism and serum level in postmenopausal osteoporosis. Journal of Trace Elements in Medicine and Biology. 2018; 48: 52-6.
- [47]Roumeliotis S, Dounousi E, Eleftheriadis T, Liakopoulos V. Association of the inactive circulating matrix Gla protein with vitamin K intake, calcification, mortality, and cardiovascular disease: a review. International journal of molecular sciences. 2019; 20(3): 628.
- [48]Murugesan A, Kumar L, Janarthanan P. Status of single nucleotide polymorphism of matrix gla protein gene (rs4236) in Nephrolithiasis: a preliminary study in indian population. International Journal of Applied and Basic Medical Research. 2018; 8(1): 38.
- [49]Petkovich M, Jones G. CYP24A1 and kidney disease. Current opinion in nephrology and hypertension. 2011; 20(4): 337-344.
- [50]Hibler EA, Klimentidis YC, Jurutka PW, Kohler LN, Lance P, Roe DJ, Thompson PA, Jacobs ET. CYP24A1 and CYP27B1 polymorphisms, concentrations of vitamin D metabolites, and odds of colorectal adenoma recurrence. Nutrition and cancer. 2015; 67(7): 1131-1341.
- [51]Colussi G, Ganon L, Penco S, De Ferrari ME, Ravera F, Querques M, Primignani P, Holtzman EJ, Dinour D. Chronic hypercalcaemia from inactivating mutations of vitamin D 24-hydroxylase (CYP24A1): implications for mineral metabolism changes in chronic renal failure. Nephrology Dialysis Transplantation. 2014; 29(3): 636-43.
- [52]Shen H, Bielak LF, Ferguson JF, Streeten EA, Yerges-Armstrong LM, Liu J, Post W, O’Connell JR, Hixson JE, Kardia SL, Sun YV. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arteriosclerosis, thrombosis, and vascular biology. 2010; 30(12): 2648-2654.
- [53]Al-Mawlah YH, Alasadi YF, Al-Darraji MN. Association between genetic polymorphisms of (Cu/ZnSOD and CAT C262T) and the risk of breast cancer. Gene Reports. 2021; 25: 101401.
- [54]Mohammed GK, Jumaa MN. Study of genetic variations of FTO gene and its relationship to obese in Iraqi population. Biochemical and Cellular Archives. 2020; 20(2): 6715-21.
- [55]Kadri E, Dhahri K, Zaafouri A, Krichen M, Rasheed M, Khirouni K, Barillé R. Ac conductivity and dielectric behavior of a− Si: H/c− Si1− yGey/p− Si thin films synthesized by molecular beam epitaxial method. Journal of Alloys and Compounds. (2017); 705, 708-713.
- [56]Azaza NB, Elleuch S, Rasheed M, Gindre D, Abid S, Barillé R, Ammar H. 3-(p-nitrophenyl) Coumarin derivatives: Synthesis, linear and nonlinear optical properties. Optical Materials. (2019); 96, 109328.
- [57]Kadri E, Krichen M, Mohammed R, Zouari A, Khirouni K. Electrical transport mechanisms in amorphous silicon/crystalline silicon germanium heterojunction solar cell: impact of passivation layer in conversion efficiency. Optical and Quantum Electronics. (2016); 48(12), 1-15.
- [58]Enneffati M, Rasheed M, Louati B, Guidara K, Barillé R. Morphology, UV–visible and ellipsometric studies of sodium lithium orthovanadate. Optical and Quantum Electronics. (2019); 51(9), 1-19.
- [59]Jalal R, Shihab S, Alhadi MA, Rasheed M. Spectral Numerical Algorithm for Solving Optimal Control Using Boubaker-Turki Operational Matrices. In Journal of Physics: Conference Series. (2020, November); (Vol. 1660, No. 1, p. 012090). IOP Publishing.
- [60]Rasheed M, Shihab S, Sabah OW. An investigation of the structural, electrical and optical properties of graphene-oxide thin films using different solvents. In Journal of Physics: Conference Series. (2021, March); (Vol. 1795, No. 1, p. 012052). IOP Publishing.