Association of monocyte-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and tumor necrosis factor-α in various stages of chronic kidney disease
Abstract
Chronic inflammation can have a considerable impact on the progression of glomerular and tubulointerstitial pathologies in chronic kidney disease (CKD). As an alternative to the early detection of inflammatory biomarkers that are most prominent in the progression of CKD, analyzing the relationship of monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), and tumor necrosis factor-α (TNF-α) in CKD is the goal of this study. This cross-sectional study involved 65 CKD subjects, consisting of 5 stages of CKD in compliance with KDIGO standards. Estimated glomerular filtration rate (eGFR) was calculated utilizing the CKD epidemiology collaboration (CKD-EPI) creatinine formulation. MLR and NLR were calculated from the differential count hematology analyzer. TNF-α was examined using the sandwich ELISA method. Random-spot urine was used for the UACR examination. There were significant differences in MLR and TNF-α at various stages of CKD, whereas NLR exhibited no significant difference at different stages of CKD. There was a significant association in MLR, NLR, and the stages of CKD. However, TNF-α was not associated with the stages of CKD. Also, MLR and NLR were not correlated with TNF-α in CKD. Furthermore, MLR, NLR, and TNF-α had no significant relationship with urine albumin-creatinine ratio (UACR). In conclusion, MLR is better for assessing CKD progression than NLR and TNF-α.
INTRODUCTION
Over the past two decades, chronic kidney disease (CKD) has been a prominent non-communicable disease that has continued to raise mortality rates globally [1]. The annual risk of death from CKD for individuals with an estimated glomerular filtration rate (eGFR) between 30-44 ml/min/1.73m² is 4.76 per 100 people [2]. CKD has a high risk of cardiovascular disease and progresses to end-stage renal disease (ESRD), which needs costly renal replacement therapy (RRT), including kidney transplantation or dialysis [2, 3]. Although there are many different and complex causes of CKD, the disease's early signs are frequently minimal and undetectable [2]. CKD is most common in old age, women, racial minorities, and those with diabetes mellitus (DM) and hypertension [1].
Regardless of the etiology of CKD, glomerulus and tubulointerstitial dysfunction are likely to cause and be a result of chronic inflammation [4]. In chronic inflammatory activation, renal cells exhibit a proinflammatory phenotype, while activated immune cells perpetuate the ongoing inflammatory process. Sustained inflammation ultimately results in renal fibrosis, which leads to the progression of CKD and is considered an irreversible condition [5]. Different cytokines and acute-phase proteins are discharged in response to inflammation, both intensifying and decreasing the inflammatory response [6].
Numerous research studies and clinical findings substantiate the proinflammatory cytokine involvement of tumor necrosis factor-alpha (TNF-α) in the pathology of acute and chronic kidney disease [7]. Decreased albumin serum levels and elevated levels of plasma fibrinogen, IL-6, and TNF-α are linked to the prevalence and severity of CKD and an accelerated decline in renal function in individuals with CKD [8, 9]. T cells, macrophages, and monocytes are the primary producers of TNF-α. TNF-α can also be produced by kidney cells, including mesangial, glomerular, endothelial, dendritic, and renal tubular cells [10, 11].
Examination of hematological inflammatory biomarkers, such as monocyte-to-lymphocyte ratio (MLR) and neutrophil-to-lymphocyte ratio (NLR), is often used to predict the outcome of cardiovascular disease or tumors, reflecting low-intensity levels of inflammation throughout the body. These markers are also widely used in various clinical problems [12]. In the case of CKD, MLR and NLR values tend to increase along with the decrease in eGFR, which describes kidney function and its stage in CKD [12]. Increased MLR is correlated with increased renal injury in type 2 diabetes mellitus (T2DM) patients, so MLR can be used to predict the incidence of diabetic nephropathy [13]. Previous research has demonstrated a positive relationship between NLR and TNF-α in patients with ESRD [14].
In the study published in 2013, a study was performed on different profiles of TNF-α and other cytokines among five different stages of CKD patients, revealing an important distinction in the kinetics of the interaction between these cytokines and kidney function [15]. The hypothesis assumes that chronic inflammation-induced long-term activation of the innate immune system exacerbates the disease and may be a major factor in the development of multiple disorders that lead to diabetic nephropathy, such as endothelial dysfunction, oxidative stress, insulin resistance, and hyperglycemia, as well as in the decline and advancement of CKD [15]. Acute myeloid-driven innate immune responses, which are reported to have chronic lymphocyte-driven immunological memory represented by lymphocyte counts, are measured by MLR and NLR [10].
The healthcare system has been confronted with the difficulty of treating patients with CKD who are either newly diagnosed or undiagnosed because of limited or absent symptoms [2]. The diagnosis is usually made when pathological results emerge after screening tests (urine dipstick or blood tests) or when symptoms become severe [16]. Numerous academic studies indicate that inflammatory mechanisms are activated during the early stages of CKD, promoting the decline of kidney function. For this reason, measuring inflammatory biomarkers could assist in the early detection of CKD [16, 17]. MLR, NLR, and TNF-α, as the most significant inflammatory indicators in various stages of CKD, have not yet been examined in any studies. So, the objective of this study is to analyze the association of MLR, NLR, and TNF-α with the stages of CKD and urine albumin-to-creatinine ratio (UACR) as an alternative to the early detection of inflammatory biomarkers that play the most important role in the progression of CKD.
MATERIALS AND METHODS
Ethical statement
This cross-sectional study was conducted at the Internal Medicine and Hemodialysis Unit of Universitas Airlangga Hospital, Surabaya, Indonesia, in mid-2023. The research subjects provided informed consent before participating in this study. This research was authorized by the Research Ethics Committee of Universitas Airlangga Hospital, Surabaya, Indonesia, No. 094/KEP/2023.
Data collection
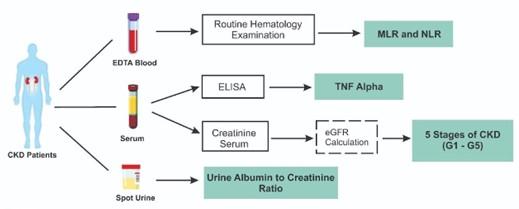
Consecutive sampling was used to acquire the data. Data were obtained from 65 subjects, consisting of five stages of CKD as defined by eGFR according to KDIGO guidelines, with the inclusion criteria of male and female subjects aged more than 18 years, diagnosed with CKD, and having a history of T2DM and/or hypertension. Exclusion criteria included subjects with cardiovascular disease, liver disorders (liver cirrhosis and/or hepatitis B or C), confirmed acute infectious diseases and acute infections, active or chronic inflammatory and autoimmune diseases, active hematological or oncological proliferative diseases and tumors, and receiving steroid and immunosuppressant therapy. Data on the study population characteristics, such as age, gender, weight, and height, which are calculated into BMI, blood pressure, hypertension, and history of diabetes, were collected using case report forms by interviewing the subject and patient medical records. The researchers' primary data included hemoglobin, eGFR, neutrophils, lymphocytes, monocytes, and proteinuria.
Analysis of urine and blood parameters
Each subject provided a blood sample of up to 3 mL, which was then collected in both EDTA and serum separation tubes. In addition, random-spot urine was collected from the subjects for UACR examination. EDTA blood samples were used for the hematology examination. Serum samples were used for serum creatinine and TNF-α examinations. Serum and urine creatinine were examined by the kinetic test without deproteinization according to the Jaffe method using the automatic clinical chemistry analyzer TMS 24i Premium instrument (Tokyo Boeki Medical System LTD., Tokyo, Japan) with Proline Creatinine FS reagent (Prodia Diagnostic Line, Bekasi, Indonesia). The eGFR was calculated using the CKD-EPI creatinine formulation [18]. A hematology examination was performed using a Sysmex XN-550 hematology analyzer instrument (Sysmex Corporation, Kobe, Japan). The MLR was calculated as monocytes divided by lymphocytes. The NLR was calculated as neutrophils divided by lymphocytes. TNF-α was examined using the Sandwich ELISA method by Human TNF-α Bioassay Technology (BT) Laboratory kit reagent (Shanghai Korain Biotech Co., Ltd., Zhejiang, China), and the optical density (OD) value was determined using a Bio-Rad iMark™ Microplate Reader (Bio-rad Laboratories Inc., Hercules, California, USA) set at 450 nm. TNF-α concentration was defined by plotting a standard curve. Urine albumin was examined using the immunoturbidimetric method by Norudia U-ALB reagent (Sekisui Medical Co., Ltd., Tokyo, Japan), and UACR was automatically calculated through the TMS 24i Premium clinical chemistry analyzer (Tokyo Boeki Medical System LTD., Tokyo, Japan). All procedures for parameter examination are conducted according to the protocol described in the manufacturer's instructions.
Statistical analysis
MLR, NLR, TNF-α, and UACR data obtained were analyzed using the Statistical Package for the Social Sciences (SPSS) 25.0 for Windows program (IBM, Armonk, NY, USA). The analysis of differences in MLR, NLR, and TNF-α in various stages of CKD used the Kruskal-Wallis test and was considered significant if the p-value was < 0.05. Analysis of the association between MLR, NLR, and TNF-α with the stages of CKD, as well as the relationship between MLR, NLR, and TNF-α with UACR using the Spearman rank correlation test, demonstrated significance if the p-value was < 0.05. Analysis of the relationship between MLR and NLR with TNF-α in CKD disease using the Spearman rank correlation test is necessary because the data is not normally distributed and is considered significant if the p-value is < 0.05. The direction and strength of the relationship are measured based on the r value (correlation coefficient).
RESULTS
Characteristics of study population
The characteristics of the 65 subjects with CKD (Table 1) indicated a mean age of 53.81 ± 8.10 years. Out of the total subjects, 34 (52.3%) were female, and 59 (90.8%) reported not having a smoking habit. Subjects in CKD stages 1 and 2 had a mean BMI of 30.19 ± 8.11, classifying them in the overweight category, whereas those in CKD stages 3, 4, and 5 had a mean BMI in the normal category. The mean SBP of CKD stage 1 subjects was the lowest at 136.8 ± 19.63, while CKD stage 5 was the highest at 148.33 ± 11.85 compared to other stages. Additionally, the mean DBP of each group of the research subjects was quite varied. Most of the subjects, 56 (86.15%), had hypertension, and 21 (32.31%) had been diagnosed with type 2 diabetes mellitus for 5–10 years. Additionally, 8 (12.31%) research subjects did not have a history of type 2 diabetes mellitus. Hemoglobin levels were highest among subjects in CKD stage 1, with a mean of 13.71 ± 1.68, while those in CKD stage 5 had the lowest mean of 9.26 ± 2.2. All subjects were grouped based on eGFR values (47 (2-117)) to determine their CKD stages according to KDIGO guidelines. The results of the UACR examination of the CKD subjects included 4 (6.14%) with normoalbuminuria (UACR < 30 mg/g), 28 (43.08%) with microalbuminuria (30 ≤ UACR ≤ 300), and 33 (50.76%) with macroalbuminuria (UACR > 300 mg/g). Table 1 presents the characteristics of the study population.
Table 1. Characteristics of the study population.
MLR, NLR, and TNF-α profile by eGFR-based stages of CKD patient categorization
A Kruskal-Willi’s test was conducted to analyze the differences in MLR, NLR, and TNF-α at various stages of CKD. The results revealed a significant difference in MLR and TNF-α at various stages of CKD with p-values of 0.003 and 0.025 (p < 0.05), respectively. However, NLR exhibited no significant difference at various stages of CKD, with a p-value of 0.345 (p > 0.05) (Table 2). MLR and TNF-α tend to increase as the degree of CKD progresses. The highest MLR value and the lowest TNF-α levels are observed in CKD stage 5.
Table 2. Analysis of differences in MLR, NLR, and TNF-α at various stages of CKD.
Association of MLR, NLR, and TNF-α with the various stages of CKD
The results of the Spearman correlation test revealed a significant positive association between MLR (p < 0.001, r = 0.470) and NLR (p = 0.046, r = 0.248) and the stage of CKD. There was no significant association between TNF-α levels and the stage of CKD (p = 0.639, r = -0.059) (Table 3).
Table 3. Correlation of MLR, NLR, and TNF-α with the stages of CKD.
Association of MLR and NLR with TNF-α at various stages of CKD
There was no association between MLR and NLR with TNF-α (p > 0.05) in chronic kidney disease, according to the results of the Spearman correlation test (Table 4).
Table 4. Correlation of MLR and NLR with TNF-α at CKD stages.
Association between MLR, NLR, and TNF-α with UACR
Spearman's rank correlation analysis indicated that MLR, NLR, and TNF-α were not significantly associated with UACR in chronic kidney disease (p > 0.05) (Table 5).
Table 5. Correlation of MLR, NLR, and TNF-α with UACR at various stages of CKD.
DISCUSSION
Factors contributing to the development of CKD include macro- and microalbuminuria, anemia, hyperkalemia, hyperphosphatemia, metabolic acidosis, stage 4 CKD, and T2DM [19]. Previous studies have indicated that risk factors such as old age, obesity, increased systolic blood pressure (SBP), hypertension, T2DM, long duration of diabetes, and family history of renal disease are significantly associated with the incidence of CKD [20]. This study is the first to analyze differences in MLR and NLR values in various stages of CKD. A study conducted by Zhang et al. stated that patients with CKD, after treatment, exhibited higher levels of inflammatory markers (leukocytes, neutrophils, monocytes, MLR, and NLR) and lower eGFR [12]. The prognosis of individuals with CKD and T2DM is closely linked to the blood system's monocytes and lymphocytes changing slowly over the course of a chronic inflammatory state [21]. The NLR, which is not significantly different in various stages of CKD, could be attributed to the specific role of neutrophils in providing specific innate immunity to prevent the spread of pathogens, serving as the first line of defense against infection, and subsequently activating adaptive immunity [22, 23].
The results of this research align with a study by Oh et al., which also analyzed differences in TNF-α at five stages of CKD and reported a statistically significant difference (p = 0.043) [15]. The study findings indicated that IL-6 is superior to TNF-α in distinguishing between several stages of CKD. Serum levels of both TNF-α and IL-6 rise as eGFR decreases and peak in patients with severe CKD [15]. Other studies have indicated that hemodialysis (HD) patients had lower levels of NLR, CRP, IL-6, and TNF-β than peritoneal dialysis (PD) patients [14]. This study observed a decrease in TNF-α in CKD stage 5, which includes hemodialysis and non-dialysis patients. Immune cells exhibited unsuitability for producing selected cytokines, possibly due to dialysis procedures or a uremic environment [24].
This study revealed that increases in MLR and NLR were associated with CKD progression. These findings are consistent with the study of Huang et al., which stated that a rise in MLR was significantly linked to the possibility of diabetic nephropathy and could serve as a reliable predictor of its occurrence [13]. The ability of monocytes to predict a decline in kidney function and the number of intermediate monocytes (CD14++, CD16+) were inversely associated with eGFR [25]. In addition to being more affordable, efficient, and simple to obtain than other conventional markers, MLR is also more stable and suitable for clinical applications than single indicators [21]. An effective method for evaluating inflammation is to combine monocytes and lymphocytes. Inflammation can respond to uremic control and act as a marker of alterations in the disease's early stages before clinical manifestations [26]. The results of this research are in line with prior studies regarding the relationship between NLR and eGFR in Chinese T2DM patients, revealing significant results and a negative correlation [27].
NLR also indicated a substantial positive relationship with creatinine in hypertensive patients, especially at high NLR values [28]. Another study concluded that the NLR level has been observed to be an independent determinant of the risk for renal disease progression in individuals with stages 1-4 of CKD, suggesting that NLR may be used to predict disease progression in CKD patients [29]. This research also demonstrated that TNF-α was not significantly correlated with the stages of CKD. These results align with prior research, stating that there was no correlation between serum TNF-α and eGFR in T2DM patients with an average duration of 14 years and an eGFR of 76.7 mL/minute/1.73 m2 [30]. Additionally, the results obtained are consistent with a study conducted by Oh et al. in CKD patients. According to the multiple regression analysis's findings, serum TNF-α is not related to eGFR (β value = -0.029; p = 0.877) [15].
This research demonstrated that MLR and NLR in CKD were not associated with TNF-α. These results differ from previous studies in which there was a positive relationship between NLR and TNF-α in patients with ESRD [14]. These different results could be attributed to different study populations, where this study involved CKD patients of various stages and did not include patients who received peritoneal dialysis therapy. The relationship of MLR and NLR to TNF-α is based on the pleiotropic action of TNF-α. TNF-α is released by immune cells, including monocytes, neutrophils, and lymphocytes, and can activate immune cells to differentiate, then migrate to the infection site and mediate the inflammatory response [30].
However, kidney cells are also capable of producing TNF-α, such as mesangial, glomerular, endothelial, dendritic, and renal tubular cells [11]. In addition, the kidneys play a role in controlling cytokine homeostasis. Research on the kidneys' function in the clearance of inflammatory cytokines in patients with sepsis observed that the kidneys remove several plasma-derived proinflammatory cytokines during diuresis, an early stage of the disease [31]. In non-proteinuric systemic inflammatory conditions, including those following heart surgery, the kidneys preferentially filter smaller proinflammatory molecules and less readily filter larger anti-inflammatory cytokines and soluble cytokine receptors [32]. Furthermore, filtered proinflammatory cytokines are absorbed in the proximal renal tubule and denatured by an intracellular process known as proteolysis rather than being eliminated intact in the urine [32]. Researchers assume that in CKD conditions, there is a decrease in glomerular filtration and reabsorption in the kidney tubules so that proinflammatory cytokines enter the urine. The condition proves that the TNF-α pathway does not only involve immune cells and is more complex, supporting the research results that there was no relationship between MLR and NLR with TNF-α.
Metalloproteinase TNF-α converting enzyme secretes TNF-α, a 26-kDa plasma membrane protein, into the extracellular space [33]. However, numerous factors may impact the levels of inflammatory biomarkers in circulation, and circulating levels of TNF-α may not reflect biological activity at the tissue level [34]. Serum TNF-α levels were observed to increase in diabetic polyneuropathy patients. These levels were negatively associated with nerve conduction velocity and positively associated with neuropathy symptoms and impairment [35]. TNF-α levels have a strong positive relationship with HbA1c and are positively related to insulin resistance [36]. In hypertension, there is a disruption of the TNF system; several previous studies indicated a decrease in TNF-α levels but an increase in tumor necrosis factor receptor 1 (TNFR1) and tumor necrosis factor receptor 2 (TNFR2). Thus, it can be concluded that an increase in sTNFR1 and sTNFR2 can be a marker of kidney dysfunction and chronic inflammation in hypertension [37-39].
The results of this study indicated a tendency wherein the UACR increases with increasing MLR, NLR, and TNF-α. Nevertheless, there was no correlation between MLR, NLR, and TNF-α with UACR at CKD. UACR is used to determine the level of albuminuria in CKD because it is more standardized and has better precision for detecting lower albuminuria values. UACR is suggested as a more precise and sensitive marker of glomerular pathology [40]. A study by Zhang et al. on CKD patients revealed that MLR could predict the risk of decreased eGFR, but it is not statistically significant to predict proteinuria [12]. Another study on T2DM patients with microalbuminuria and normoalbuminuria indicated that MLR results are associated with microalbuminuria (r = 0.228, p = 0.001). However, the coefficient correlation value revealed that the level of correlation is very weak [41].
This study's results differ from studies regarding the association between NLR and UACR in Chinese diabetic kidney disease patients with T2DM conducted by Li et al., revealing that the NLR on the levels of albuminuria differed significantly and had a positive correlation [27]. However, this is in line with Chollangi et al., who stated that there was no correlation between NLR and UACR in both groups of uncontrolled DM patients (HBA1c > 7%) with microalbuminuria and without microalbuminuria, but there is a tendency that the higher the NLR, the higher the UACR [42]. There have also been reports of increased neutrophils' spontaneous attachment to endothelial cells as a potential explanation for proteinuria [43]. The reason for the different results lies in the wide range of UACR values, spanning from the lowest to the highest, rendering it statistically insignificant. Of the entire CKD population, only four patients had normoalbuminuria and were included in the analysis.
This study's results are also in line with the research of Lampropulou et al., which stated that serum levels of TNF-α were not correlated with the degree of UACR or the severity of the deterioration in kidney function but correlated significantly with CRP, serum levels of TNFR2, urine TNF-α, and were weaker in TNFR1 [44]. Research conducted by Lampropoulou et al. stated that TNF-α levels in the urine and serum did not correlate with one another; this suggests that the production of this cytokine is mainly intrarenal, and therefore, inflammatory activation is local, and the inflammatory response is non-systemic [30]. Albuminuria could potentially arise from the proliferation and expansion of mesangial, which might be triggered by TNFR1. Elevating soluble TNFR1 and TNFR2 levels in serum might hasten the decline of kidney function, even in the early stages without proteinuria, suggesting the possibility of another undefined pathway of TNF-α and its receptors for proteinuria [44].
This study had several limitations, including the use of subjects from various CKD etiologies, where the characteristics of the research subjects included patients with a history of T2DM and/or hypertension. Researchers did not exclude T2DM patients with multiple complications, for example, diabetic neuropathy and retinopathy, and researchers did not measure glycemic control in diabetes mellitus patients through HbA1C examination.
CONCLUSION
The current study reveals that MLR exhibits significant differences and a positive correlation with CKD stages (Figure 1). In contrast, NLR and TNF-α do not exhibit differences or correlations based on CKD stages. Therefore, MLR can be considered the inflammatory marker that plays the most significant role in CKD progression. Further research is needed, considering the sample size and other factors influencing inflammation in CKD. Additionally, further investigations are required to assess the ability of MLR and NLR as simple and inexpensive inflammatory markers compared to other more specific inflammatory markers.
ACKNOWLEDGMENT
The authors express their gratitude to the Universitas Airlangga, the Faculty of Medicine, as well as employees and study participants at the Universitas Airlangga Hospital. Additional appreciation from the authors is extended to the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia, with contract number 1185/UN3.LPPM/PT.01.03/2023, for providing financial support for the implementation of this research.
AUTHOR CONTRIBUTIONS
DIS, PW, and YP participated in the conception and planning of the research, with PW taking part in supervising the work. YP and SDS performed the measurements and data acquisition/collection. DIS and PW conducted the statistical analysis. DIS, PW, and YP drafted the manuscript and interpreted the results. Each author contributed to the manuscript's critical editing.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Kovesdy CP. Epidemiology of chronic kidney disease: An update 2022. Kidney International Supplements. 2022;12:7-11.
- [2]Borg R, Carlson N, et al. The growing challenge of chronic kidney disease: An overview of current knowledge. International Journal of Nephrology. 2023;2023.
- [3]Ying M, Shao X, et al. Disease burden and epidemiological trends of chronic kidney disease at the global, regional, national levels from 1990 to 2019. Nephron. 2023.
- [4]Stenvinkel P, Chertow GM, et al. Chronic inflammation in chronic kidney disease progression: Role of nrf2. Kidney international reports. 2021;6:1775-87.
- [5]Lousa I, Reis F, et al. The signaling pathway of tnf receptors: Linking animal models of renal disease to human ckd. International Journal of Molecular Sciences. 2022;23:3284.
- [6]Gupta J, Mitra N, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in ckd in cric. Clinical journal of the American Society of Nephrology: CJASN. 2012;7:1938.
- [7]Vielhauer V, Mayadas TN. Functions of tnf and its receptors in renal disease: Distinct roles in inflammatory tissue injury and immune regulation. Seminars in nephrology: Elsevier; 2007. p. 286-308.
- [8]Lee BT, Ahmed FA, et al. Association of c-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC nephrology. 2015;16:1-6.
- [9]Amdur RL, Feldman HI, et al. Inflammation and progression of ckd: The cric study. Clinical journal of the American Society of Nephrology: CJASN. 2016;11:1546.
- [10]Mureșan AV, Russu E, et al. The predictive value of nlr, mlr, and plr in the outcome of end-stage kidney disease patients. Biomedicines. 2022;10:1272.
- [11]Nogueira A, Pires MJ, et al. Pathophysiological mechanisms of renal fibrosis: A review of animal models and therapeutic strategies. In vivo. 2017;31:1-22.
- [12]Zhang M, Wang K, et al. Monocyte lymphocyte ratio predicts the new-onset of chronic kidney disease: A cohort study. Clinica Chimica Acta. 2020;503:181-9.
- [13]Huang Q, Wu H, et al. Monocyte–lymphocyte ratio is a valuable predictor for diabetic nephropathy in patients with type 2 diabetes. Medicine. 2020;99.
- [14]Turkmen K, Guney I, et al. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Renal failure. 2012;34:155-9.
- [15]Oh D-J, Kim HR, et al. Proflie of human β-defensins 1, 2 and proinflammatory cytokines (tnf-α, il-6) in patients with chronic kidney disease. Kidney and Blood Pressure Research. 2013;37:602-10.
- [16]Webster AC, Nagler EV, et al. Chronic kidney disease. The lancet. 2017;389:1238-52.
- [17]Schlondorff DO. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney international. 2008;74:860-6.
- [18]Rovin BH, Adler SG, et al. Kdigo 2021 clinical practice guideline for the management of glomerular diseases. Kidney international. 2021;100:S1-S276.
- [19]Sui Z, Wang J, et al. Aetiology of chronic kidney disease and risk factors for disease progression in chinese subjects: A single‐centre retrospective study in beijing. Nephrology. 2020;25:714-22.
- [20]Damtie S, Biadgo B, et al. Chronic kidney disease and associated risk factors assessment among diabetes mellitus patients at a tertiary hospital, northwest ethiopia. Ethiopian journal of health sciences. 2018;28.
- [21]Qiu C, Liu S, et al. Prognostic value of monocyte-to-lymphocyte ratio for 90-day all-cause mortality in type 2 diabetes mellitus patients with chronic kidney disease. Scientific Reports. 2023;13:13136.
- [22]Döring Y, Soehnlein O, et al. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circulation research. 2017;120:736-43.
- [23]Djordjevic D, Rondovic G, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: Which ratio to choose to predict outcome and nature of bacteremia? Mediators of inflammation. 2018;2018.
- [24]Mansouri L, Paulsson JM, et al. Leukocyte proliferation and immune modulator production in patients with chronic kidney disease. PloS one. 2013;8:e73141.
- [25]Naicker SD, Cormican S, et al. Chronic kidney disease severity is associated with selective expansion of a distinctive intermediate monocyte subpopulation. Frontiers in immunology. 2018;9:2845.
- [26]Xiang F, Chen R, et al. Monocyte/lymphocyte ratio as a better predictor of cardiovascular and all‐cause mortality in hemodialysis patients: A prospective cohort study. Hemodialysis International. 2018;22:82-92.
- [27]Li L, Shen Q, et al. Association of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with diabetic kidney disease in chinese patients with type 2 diabetes: A cross-sectional study. Therapeutics and Clinical Risk Management. 2022:1157-66.
- [28]Chen C, Zhao HY, et al. Correlation between neutrophil‐to‐lymphocyte ratio and kidney dysfunction in undiagnosed hypertensive population from general health checkup. The Journal of Clinical Hypertension. 2020;22:47-56.
- [29]Yoshitomi R, Nakayama M, et al. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in japanese patients with chronic kidney disease. Renal failure. 2019;41:238-43.
- [30]Lampropoulou I-T, Stangou M, et al. Tnf-α and microalbuminuria in patients with type 2 diabetes mellitus. Journal of diabetes research. 2014;2014.
- [31]Graziani G, Bordone G, et al. Role of the kidney in plasma cytokine removal in sepsis syndrome: A pilot study. Journal of Nephrology. 2006;19:176-82.
- [32]Cackovic M, Buhimschi CS, et al. Fractional excretion of tumor necrosis factor-α in women with severe preeclampsia. Obstetrics and gynecology. 2008;112:93.
- [33]Ramseyer VD, Garvin JL. Tumor necrosis factor-α: Regulation of renal function and blood pressure. American Journal of Physiology-Renal Physiology. 2013;304:F1231-F42.
- [34]Stenvinkel P, Ketteler M, et al. Il-10, il-6, and tnf-α: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney international. 2005;67:1216-33.
- [35]El Sheikh WM, Alahmar IE, et al. Tumor necrosis factor alpha in peripheral neuropathy in type 2 diabetes mellitus. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery. 2019;55:1-7.
- [36]Alzamil H. Elevated serum tnf-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. Journal of obesity. 2020;2020.
- [37]Gohda T, Niewczas MA, et al. Circulating tnf receptors 1 and 2 predict stage 3 ckd in type 1 diabetes. Journal of the American Society of Nephrology: JASN. 2012;23:516.
- [38]Niewczas MA, Gohda T, et al. Circulating tnf receptors 1 and 2 predict esrd in type 2 diabetes. Journal of the American Society of Nephrology: JASN. 2012;23:507.
- [39]Eguchi T, Maruyama T, et al. Disturbed tumor necrosis factor system is linked with lower egfr and chronic inflammation in hypertension. The International Journal of Biological Markers. 2014;29:e69-e77.
- [40]Chen TK, Knicely DH, et al. Chronic kidney disease diagnosis and management: A review. Jama. 2019;322:1294-304.
- [41]Kocak MZ, Aktas G, et al. Monocyte lymphocyte ratio as a predictor of diabetic kidney injury in type 2 diabetes mellitus; the madkid study. Journal of Diabetes & Metabolic Disorders. 2020;19:997-1002.
- [42]Chollangi S, Rout NK, et al. Exploring the correlates of hematological parameters with early diabetic nephropathy in type 2 diabetes mellitus. Cureus. 2023;15.
- [43]Huang L, Xie Y, et al. Neutrophil-to-lymphocyte ratio in diabetic microangiopathy. Int J Clin Exp Pathol. 2017;10:1223-32.
- [44]Lampropoulou IT, Stangou Μ, et al. Tnf-α pathway and t-cell immunity are activated early during the development of diabetic nephropathy in type ii diabetes mellitus. Clinical Immunology. 2020;215:108423.