Oxidant and antioxidant status of erythrocytes and plasma samples in polycystic ovary syndrome patients
Abstract
Polycystic ovary syndrome (PCOS) is one of the most common types of endocrine syndromes in women, where the disease is described as chronic anovulation and hyperandrogenism, which was characterized by the formation of small cysts (fluid-filled sacs) in the ovaries. This study was carried out to introduce knowledge when plasma and erythrocytes portions were chosen to compare the antioxidant and oxidant status of PCOS patients treated or without treatment with metformin. Samples included 75 women diagnosed with PCOS according to Rotterdam criteria. The control group included healthy volunteers with regular menstrual cycles and with no symptoms of hyperandrogenism. Total antioxidants capacity (TAC), total oxidant status (TOS), and Cu\Zn SOD enzyme activity were examined in both plasma and erythrocytes. The activity of the Cu\Zn SOD enzyme was determined based on the ability of the enzyme to inhibit the autoxidation of pyrogallol. Body mass index (BMI), hormonal tests (LH and FSH) showed significant differences in PCOS patients as compared to control. In both plasma and erythrocyte, TAC levels were significantly decreased in PCOS patients. Though the activity of superoxide dismutase was significantly higher in plasma and erythrocytes in patients. On the other hand, PCOS patients showed significantly higher levels of TOS in plasma, while erythrocytes did not show any significant differences. In addition, metformin treatment significantly decreased levels of TAC, TOS and SOD1 activity in PCOS patients. Considering all, it is concluded that both TOS and SOD1 activity introduce the same pattern in blood samples, TAC displays a different line of responses which can be due to the crucial role of SOD1 as an antioxidant factor.
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is one of the most widespread, frequent and complicated endocrinological disorders in adolescents and women of reproductive age [1]. By an ESHRE/ASRM consensus or so-called Rotterdam criteria in 2003 [2], this disease is characterized by the presence of two or more features including both reproductive and metabolic characteristics, biochemical elevated androgen, persistent oligo-ovulation or anovulation, and polycystic ovaries. Hyperandrogenism, pregnancy problems, ovarian dysfunction, and infertility are all examples of reproductive issues. Whereas insulin resistance, dyslipidemia, reduced glucose tolerance, and diabetes mellitus are among the metabolic consequences [3].
The development of PCOS has also been linked to oxidative stress and low-grade inflammation [4]. Oxidative stress status results from an imbalance between the systemic expression of reactive oxygen species (ROS) and the biological system’s inability to quickly detoxify or repair the reactive intermediates, resulting in peroxides of lipids, DNA, and proteins [5]. Increased oxidative stress and reduced antioxidant capacity are thought to be linked with more than clinical disorders, for instance, increased risk of cardiovascular disease, hypertension, central obesity and dyslipidemia, and insulin resistance [6]. PCOS has been linked to oxidative stress by meta-analysis research and identified the role of the abnormal level of circulating oxidative stress markers in the pathophysiology of PCOS patients [7,8]. Physical and antioxidant defenses in addition to Preventative and repair processes are among the physiological mechanisms that protect the body against the oxidant species [9]. Antioxidants are substances that can neutralize, scavenge, or prevent the generation of oxidants. These antioxidants include enzymatic (superoxide dismutase (SOD), catalase, glutathione peroxidase, and paraoxonase) and non-enzymatic materials such as ascorbic acid, glutathione and vitamin E [10]. SOD is responsible for catalyzing the conversion of superoxide oxygen and hydrogen peroxide by dismutation reaction, in which one molecule is reduced and the other is oxidized; in this case, two molecules of superoxide anions were transformed into hydrogen peroxide and molecular oxygen. Humans have three different types of superoxide dismutase. SOD1 is found in the cytoplasm, SOD2 is found in the mitochondria, and SOD3 is found outside the cell. The first is a dimer (two units), whereas the others are tetramers (four units) (four subunits). Copper and zinc are found in SOD1 and SOD3, but manganese is found in SOD2, a mitochondrial enzyme [11]. The aim of this study is to evaluate the oxidant and antioxidant status for patients diagnosed with PCOS by quantification of total oxidant and total antioxidant particularly the antioxidant enzyme activity of Cu\Zn SOD in plasma and erythrocyte portions.
MATERIALS AND METHODS
Sample collection
This study was performed during the period from November 2019 until October 2021, the samples were collected from seventy-five females (married and unmarried/ patients and controls) who regularly visited the AL- Sadiq Hospital and private gynaecology clinic in AL- Hillah city. The age of participants ranged from 20 to 45 years old, and their historical data were conducted by collecting information related to standard symptoms such as acne, amenorrhea, and excessive hair growth. Their residence (urban, rural), risk factors (obesity, hereditary factors), other past medical diseases (diabetes, hypertension, asthma, epilepsy) and their hormonal tests (FSH and LH) were all recorded.
Rotterdam criteria
The Rotterdam criteria were used to diagnose the symptoms of PCOS disease since all the participants were signed written consent and fully informed about the study methodology. Adrenal congenital hyperplasia, cushing syndrome, and androgen-secreting tumors, as well as other medical conditions, were excluded from the study. The women in the control group all have regular menstrual cycles lasting 4-5 days and regularity of 25-30 days, as well as no symptoms of increased androgen.
Ethical statement
Written permissions were taken by volunteers before taking samples for research and the procedures for this research were carried out under the ethical approval numbered (DSM/HO-15314) for scientific research from the ethics committees of the Ministry of Higher Education and Scientific Research and the Iraqi Ministry of Health.
Hormonal tests
In this experiment, anti-luteinizing hormone (LH) and anti-follicle stimulating hormone (FSH) monoclonal antibodies were used to label ABEI and then another monoclonal antibody was used to make the sample to be diagnosed bound to both sides of the antibodies. The sample was then incubated on the pallet at 37 °C for half an hour and then g, to form a sandwich; After sedimentation in the field of magnets Celt once with the washing solution, then, the substrate 1 and 2 were added to reach the immune-chemiluminescence reaction, finally the light signal was measured by a photomultiplier such as RLU within 3 seconds and the amount of the emitted immunofluorescence increased directly with the concentration of LH and FSH present in the samples.
Activity of Cu\Zn SOD enzyme
(Cu\Zn) SOD enzyme activity was determined by used a simple and rapid method based on the ability of the enzyme to inhibit the autoxidation of pyrogallol. The autoxidation of pyrogallol in the presence of EDTA in pH (8.2) is 50%. The principle of this method is based on the competition between pyrogallol autoxidation by O2•¯ and the dismutation of this radical by SOD (Cu\Zn) SOD activity is expressed as units/ml. One unit of (Cu/Zn) SOD activity is defined as the amount of enzyme required to cause 50% inhibition of pyrogallol autoxidation [12,13].
TOS of plasma was measured using a method developed by Erel [14]. Oxidants in the plasma oxidize the ferrous ion–o-dianisidine complex to ferric ion. The oxidation reaction is improved by glycerol molecules abundantly found in the reaction medium. The ferric ion creates a colored complex with xylenol orange in an acidic medium. Color intensity, which can be determined spectrophotometrically, is associated with the sample’s total amount of oxidant molecules. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (µmol H2O2 Eq/L).
Antioxidants + Cu+2 = Cu+
Cu+ + 2, 9-dimethyl-1, 10-phenanthroline > complex (λ max at 450 nm)
Statistical analysis
Most of the experiments were statistically analyzed using a one-way analysis of variances (ANOVA) and the t-test. Experiments were carried out in triplicate and error bars represent the standard deviation (±) of the means. Analysis was performed using GraphPad Prism7 software. Experiments were carried out in triplicate and error bars represent the standard deviation (±) of the means. Analysis was performed using GraphPad Prism7 software [15].
RESULTS
Distribution and characteristics of the participants
Table 1 showed the association between PCOS and the control group according to the age, body mass index (BMI), hormonal tests (LH and FSH), history of infertility, hyperandrogenism, and menstrual cycle. Whereas BMI, FSH and LH showed significant differences (p ≤0.01) in PCOS patients as compared to control (Table 1).
Table 1. The association between PCOS patients and control group according to the age, body mass index (BMI), hormonal tests (LH and FSH), history of infertility, hyperandrogenism, and menstrual cycle.
Level of total antioxidants capacity in PCOS
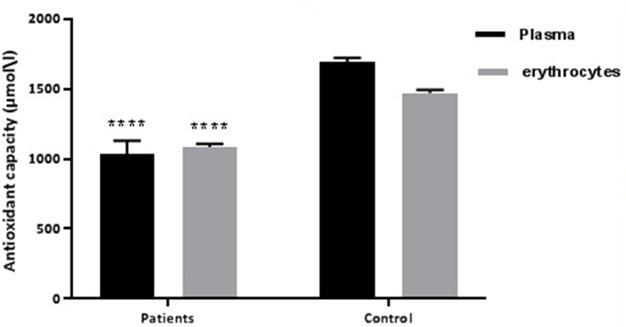
Figure 1 showed the relationship of TAC in plasma and erythrocytes, where the PCOS patients showed significantly lower level (p≤0.01) of TAC in both plasma and erythrocytes.
Level of total oxidant status in PCOS
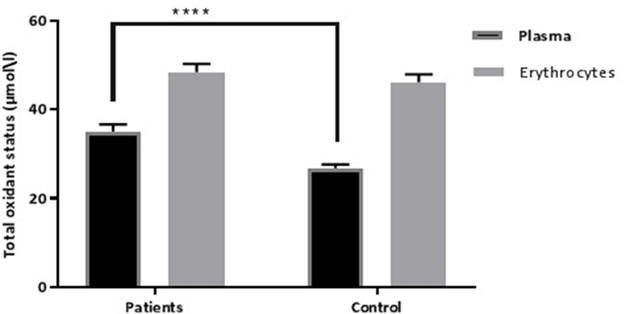
Figure 2 showed the relationship of TOS in plasma and erythrocytes. PCOS patients showed significantly higher levels (p≤0.01) of TOS in plasma samples, while erythrocytes didn’t show any significant differences (Figure 2).
Cu\Zn superoxide dismutase enzyme activity in PCOS
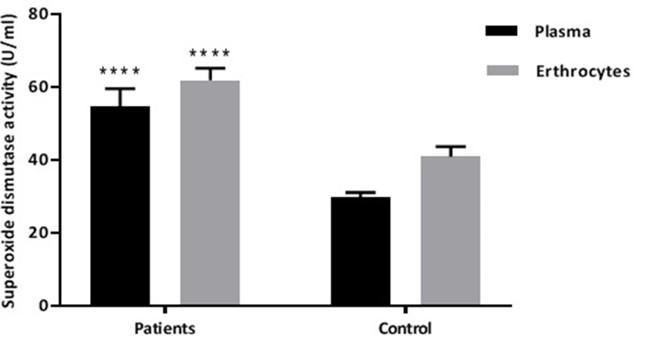
Figure 3 showed the Cu\Zn superoxide dismutase enzyme activity in both PCOS patients and control group according to the plasma and erythrocytes, where these two parameters showed significantly higher levels (p ≤0.01) of activity in PCOS patients.
Effect of metformin on antioxidant and oxidant status
Table 2 showed the effect of metformin on antioxidant and oxidant status in both PCOS patients and the control group according to the plasma and erythrocytes. The plasma and erythrocytes according to the Cu/Zn SOD activity showed significant differences at 0.0196 and 0.0450, respectively, while TAC showed significant differences only in plasma at 0.0486. Finally, according to the TOS showed significant differences in both plasma and erythrocytes at 0.0308 and 0.0050, respectively.
Table 2. Effect of metformin on antioxidant and oxidant status in both PCOS patients and control group according to the plasma and erythrocytes.
DISCUSSION
The analysis of the total oxidant, antioxidant and SOD1 activity in patients diagnosed with PCOS in considering with treatment by metformin medication was to present valuable information that can visualize their interactions. This study presented data considering for the first time the knowledge that can be extracted when samples of erythrocytes and plasma portion have been compared for the relation between oxidative stress and Metformin drug in PCOS patients. PCOS symptoms were diagnosed in 75 patients under the supervision of a Gynecologist senior doctor. According to the values in Table 1 the BMI and LH were increased significantly in patient’s diagnostic with PCOS compared with the controls group, whereas FH level was significantly decreased. These results were agreed with the finding, which was shown to be as high as 78.7% in one research, which might be due to ovaries-thyroid and pituitary axis malfunction. PCOS affects the majority of women who have irregular menstrual periods [17].
The antioxidant status was investigated, and the TAC values were significantly lower in PCOS patients than in controls, the content of TAC in plasma was significantly higher than in erythrocytes when compared to the control group of the study. Antioxidants present at high concentrations in the plasma, and these include uric acid, albumin, vitamins C and E, carotene, thiol groups, tocopherol, bilirubin and antioxidant enzymes, which are sensitive to the change in the degrees of oxidative stress [18].
TAC is considered a biomarker that measures the antioxidant potential of body fluids and has been reported to be significantly low in numerous diseases [19]. A study adapted by Hilali et al. [7] reported a deteriorating in the TAC level in patients with PCOS than the controls group. Following the oxidative stress condition, the TOS values were significantly higher in the plasma of the patient’s group than control. Moreover, the values were significantly higher in erythrocytes than in plasma within the patients and controls group. These results were agreed with previous research [20] referring to an increase in oxidative stress of plasma for PCOS patients than controls.
The higher level of oxidative stress that contributes in PCOS along with the reduction of antioxidants was similarly demonstrated in a study by Moti et al [21]. The primary role of RBCs is to transport haemoglobin, that provides oxygen to each tissue. The interaction among several factors, including hemoglobin (active metal protein) that acts as an oxidase and peroxidase, higher levels of oxygen in the circulation, membrane-bound proteins, and oxygenated unsaturated fatty acids, all provide a harmful potential environment for RBCs [22]. The later knowledge can explain to some extent the higher activity of enzyme superoxide dismutase in erythrocytes than in plasma. Although the TAC values were reported to be decreased in patients, The activity (U/ml) of superoxide dismutase was significantly higher in patients’ samples than the controls group. These results are agreed with previous studies that indicated an increase in the SOD1 activity in serum [23,24] and erythrocytes [25] and in patients with PCOS compared to control by using the pyrogallol method [26].
In considering with metformin effect on previous criteria as this medication is crucially dependent on PCOS patients’ treatment, this drug was significantly decreased the levels of total antioxidant and oxidant status along with superoxide dismutase activity when patients were compared with the controls group. The scavenging capacity of metformin against reactive oxygen species like hydroxyl, hydrogen peroxide (H2O2) and superoxide (O2.) radicals have been studied by Khouri et al [27], the authors concluded that metformin does not scavenge O2 radicals nor H2O2 but can react with OH radicals and decrees oxidative. This study partially agreed with who found lower enzyme activity in the treated group than the untreated group, but the data were not at significant levels.
CONCLUSION
In conclusion, there was statistically a significant difference according to the BMI, LH, and FSH in contrast to the other markers in PCOS patients. Also, there was an increase in the total capacity of antioxidants. The level of total oxidant status in PCOS was elevated in the plasma samples compared to the erythrocytes. Also, Cu\Zn superoxide dismutase enzyme activity in PCOS showed statistically a significant difference in both erythrocytes and plasma samples. Finally, both TOS and SOD1 activity presented the same pattern in blood samples, and TAC values showed a different line of responses, and this may be due to the critical role of SOD1 as an antioxidant factor.
ACKNOWLEDGEMENT
The authors would like to thank Dr Yasir Haider Al-Mawlah and Dr Ameer Mezher Hadi (DNA Research Center, University of Babylon, and the collage of sciences for their kind support with all laboratory equipment and providing the suitable facilities, also for drafting the manuscript to make this work done.
AUTHOR CONTRIBUTIONS
Conception and design of the study; ASN. Drafting the manuscript; ZHO. Analysis and/or interpretation of data; QIS. All authors read and approved the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Azziz, R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. The Journal of Clinical Endocrinology & Metabolism. 2004; 89(6): 2745-2749.
- [2]ESHRE. The Rotterdam, and ASRM-Sponsored PCOS Consensus Workshop Group. “Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and sterility. 2004; 81(1): 19-25.
- [3]Fatima Q, Amin S, Kawa IA, Jeelani H, Manzoor S, Rizvi SM, et al. Evaluation of antioxidant defense markers in relation to hormonal and insulin parameters in women with polycystic ovary syndrome (PCOS): a case-control study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019; 13(3): 1957-1961.
- [4]Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Current opinion in obstetrics and gynecology. 2006; 18(3): 325-332.
- [5]Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation research.2010;107(9): 1058-1070.
- [6]Mohan SK, Priya VV. Lipid peroxidation, glutathione, ascorbic acid, vitamin E, antioxidant enzyme and serum homocysteine status in patients with polycystic ovary syndrome. Biology and Medicine.2009; 1(3): 44-49.
- [7]Hilali N, Vural M, Camuzcuoglu H, Camuzcuoglu A, Aksoy N. Increased prolidase activity and oxidative stress in PCOS. Clinical endocrinology. 2013; 79(1): 105-110.
- [8]Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Human reproduction update. 2013; 19(3): 268-288.
- [9]Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007; 39(1): 44-84.
- [10]Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007; 39(1): 44-84.
- [11]Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arteriosclerosis, thrombosis, and vascular biology. 2004; 24(8): 1367-1373.
- [12]Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European journal of biochemistry.1974; 47(3): 469-474.
- [13]Apak R, Güçlü K, Özyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. Journal of agricultural and food chemistry.2004; 52(26):7970-7981.
- [14]Erel O. A new automated colorimetric method for measuring total oxidant status. Clinical biochemistry .2005; 38(12): 1103-1111.
- [15]George D, Mallery P. SPSS for Windows step by step: answers to selected exercises. A simple guide and reference. 2003; 63(1): 1461-1470.1
- [16]Alsaadi YL, Mohamad BJ. Prevalence of hyperandrogenism in Iraqi women with polycystic ovary syndrome. Iraqi Journal of Science. 2019;2600-2608.
- [17]Singla R, Gupta Y, Khemani M, Aggarwal S. Thyroid disorders and polycystic ovary syndrome: An emerging relationship. Indian journal of endocrinology and metabolism. 2015; 19(1): 25.
- [18]Garibaldi S, Valentini S, Aragno I, Pronzato MA, Traverso N, Odetti, P. Plasma protein oxidation and antioxidant defense during aging. International journal for vitamin and nutrition research. 2001; 71(6): 332-338.
- [19]Morera-Fumero AL, Diaz-Mesa E, Abreu-Gonzalez P, Fernandez-Lopez L, del Rosario Cejas-Mendez M. Low levels of serum total antioxidant capacity and presence at admission and absence at discharge of a day/night change as a marker of acute paranoid schizophrenia relapse. Psychiatry research.2017; 249: 200-205.
- [20]Verit FF, Erel O. Oxidative stress in nonobese women with polycystic ovary syndrome: correlations with endocrine and screening parameters. Gynecologic and obstetric investigation.2008; 65(4): 233-239.
- [21]Moti M, Amini L, Ardakani SS, Kamalzadeh S, Masoomikarimi M. Oxidative stress and antioxidant defense system in Iranian women with polycystic ovary syndrome. Iranian journal of reproductive medicine.2015; 13(6): 373.
- [22]Gorressen S, Stern M, van de Sandt AM, Cortese-Krott MM, Ohlig J, Rassaf T, et al. Circulating NOS3 modulates left ventricular remodeling following reperfused myocardial infarction. PloS one.2015;10(4): e0120961.
- [23]Ali AL-Dahhan NA, Albdairi AJ, Hamad AJ. Assessment of Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, Oxidative Stress and Antioxidants levels in Polycystic Ovary Syndrome Patients with Low-Grade Chronic Inflammation. Medico-Legal Update. 2021; 21(1).
- [24]Peskin AV, Winterbourn CC. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radical Biology and Medicine; 2017; 103: 188-191.
- [25]Al-Azzawie HF, Humadi EH. Oxidative Stress and the Antioxidant Mechanisms in a Sample of Iraqi Patients with Polycystic Ovary Syndrome (POS). IRAQI JOURNALOF COMMUNITY MEDICINE.2010; 23(3).
- [26]Sumithra NU, Lakshmi RL, Leela Menon N, Subhakumari KN, Sheejamol V S. Evaluation of oxidative stress and hsCRP in polycystic ovarian syndrome in a tertiary care hospital. Indian journal of clinical biochemistry.2015;30(2): 161-166.
- [27]Khouri H, Collin F, Bonnefont‐Rousselot D, Legrand A, Jore D, Gardès‐Albert M. Radical‐induced oxidation of metformin. European journal of biochemistry.2004; 271(23‐24): 4745-4752.
- [28]TASKÖYLÜ BY, Fenkci MS, ÖZTEKİN Ö, Yaşar EN, Topsakal S, Yaylali GF. How to Effect of Metformin Adding to Cyproterone Acetate+ Ethinyl Estradiol Treatment on Antioxidant Status in Polycystıc Ovary Syndrome?. Eurasian Journal of Medical Investigation. 2019; 3(1): 14-18.