Assessment of antiproliferative and toxic effects of a peptide from Momordica dioica using in vitro and in vivo studies
Abstract
Inflammation occurs during a cascade reaction to cause damage to the tissues. Increased oxidant and cytokine expression were observed in the damaged tissues. Inflammatory bowel disease (IBD) may be a chronic and lethal disease of inflammation in gastro enteric tissue characterized by intestinal inflammation. The objective of the study was to investigate the effects of the peptide from Momordica dioica in treating ulcerative colitis. The isolated protein was digested with trypsin enzyme and the hydrolyzed peptide were analyzed using LCMS where the peak obtained at 678.67Da at 17mins and MALDI-TOF analysis which showed similar peaks at 3388.7Da. The results from the MTT assay showed the IC50 value of the peptide at 100µg/mL. Similarly, the acridine orange staining showed decreased green-fluorescent nuclei cells than red fluorescent acidic vesicular organelles indicating autophagy-dependent cell death. The peptide displayed a cell viability effect on Colo-205 cells at 100 μg/mL when compared to the control. In the acute toxicity test, the mice were orally receiving peptide at a dose of 50-250 mg/kg BW for 14 days. The significant adverse effects were evident at a dose of 250mg/kg BW indicating that the LD50 value is lesser than 250 mg/kg. Based on the results obtained from this study, the peptide with a molecular mass of 3388.7Da exhibited an increased rate of cytotoxicity and autophagy induction in cancer cells at the concentration of 100 μg/mL. Therefore, the peptide can be further used for the formulation of anticancer drug for treating ulcerative colitis conditions.
INTRODUCTION
Inflammatory bowel disease (IBD) is chronic inflammation of the gastrointestinal tract and termed for certain conditions such as Crohn’s disease [1], ulcerative colitis, and other conditions [2,3]. Although both the conditions have similar characteristics, they differ in the position and nature of the inflammation. Crohn’s disease is observed with inflammation in the gastrointestinal tract, while ulcerative colitis is shown by inflammation localized to the large intestine [4]. The intestinal inflammation of the mucosa in IBD is characterized by pain in the abdominal region, frequent bloody and watery stools, loss of weight, and the intrusion of neutrophils and macrophages that are responsible for cytokine production, proteolytic enzymes, and free radicals that result in swelling and ulceration [5]. In contrast, ulcerative colitis occurs in periodic patterns and involves the rectum and entire colon. The pathological condition of ulcerative colitis is in the submucosa and mucosa crypt abscesses [6]. Inflammation is a reaction that causes damage to tissues resulting in increased expression of cytokines in the tissues. Peptide from various sources could be used as therapies to treat inflammation. An alternate treatment supported by the utilization of bioactive peptide as medicinal drug agents is being developed [7]. The peptide, in relation to little molecules, possess higher efficiency and property for their targets, primarily owing to their biological diversity [8]. Bioactive peptides have a sequence range of 5-20 amino acids. The major anti-inflammatory effect of the peptide is due to the high presence of hydrophobic and positively charged amino acids. The biological activity of the peptide mainly depends on the length, the molecular weight of the peptide, and the residues present at both ends of the peptide chain [9]. The proteins known as autophagy-related (ATG) are necessary for the autophagy process, and some of which belong to the Atg8 (autophagy-related 8) family. The proteins of the Atg8 family have all been linked to a variety of pathways, including cellular trafficking, autophagy, and cancer [10]. Improper functioning of these related proteins or dysfunction leads to various disease conditions like inflammation, homeostasis of colonic inflammation, and other disorders [11].
MATERIALS AND METHODS
Materials
Roswell Park memorial Institute medium 1640 (RPMI 1640),50X Antibiotic-Antimycotic solution-A002, Fetal bovine serum (FBS)-RM10432,1X Trypsin-EDTA solution-TCL007 was purchased from HIMEDIA Laboratories. Acridine orange, MTT, Trypsin enzyme, sodium dihydrogen phosphate, and disodium hydrogen phosphate were obtained from Sisco Research Laboratories. COLO 205 (human epithelial, colon cancer) cell lines were obtained from NCCS, PUNE, and cultured in RPMI Medium containing 5% FBS and 1% antibiotic solution at 370C maintained in a 5% CO2 Incubator.
Protein extraction
The seeds of Momordica dioica were shade dried and powdered. 10g of powdered seed was dissolved with 0.2M phosphate buffer solution (pH- 6.6) and incubated overnight in a shaker at 370C. The solution was filtered through the muslin cloth and the filtrate was centrifuged at 12000 rpm for 10 min. Then the supernatant collected was lyophilized and stored for further analysis [12].
The lyophilized powder was digested with trypsin enzyme for 2.5h at 370C with a substrate to enzyme ratio of 100:1 w/w in 0.1M sodium phosphate buffer, then the enzyme was inactivated by heating the solution at 900C for 5min. The digested solution was centrifuged at 12,000 rpm for 10min, and the supernatant was lyophilized and stored for future use [13]. The digested solution of Momordica dioica seed solution was fractionated through the 2KDa dialysis membrane against water for 24h in a magnetic stirrer overnight. The dialysate was then further lyophilized and stored at -200C for further analysis.
HPLC-MS analysis of partially purified peptide
The Mass spectrometry of the peptide mixture was analyzed using Impact HD, Bruker Germany. The column used was the zorbax eclipse C18 column with 3.5-micron Agilent, USA. The mobile Phase A: 0.1%formic acid and mobile phase B: 0.1% FA in 80% acetonitrile the mobile phase B is operated at a gradient range of 2 to 35%, then increased linearly to 80%. The flow rate of the system is maintained at 0.3 mL/min. The range was set to 200-2000 m/z [14,15].
MALDI-TOF analysis
The MALDI-TOF analysis was performed using MALDI TOF (UltrafleXtreme II) BRUKER Germany. The mobile phase was prepared by mixing 100mg of α-cyano-4-hydroxycinnamic acid in 10 mL of 1:1 acetonitrile and TFA. The sample for analysis was then mixed with the CHCA solution for analysis [16].
Cell viability assay
The viability of the cells was measured using MTT [17]. The colo205 cells were seeded at 5x 10 cells /well in 96 well plates and incubated at 370C for 24h. After 24 h the cells were treated with different concentrations of the peptide ranging from (25-125 µg/mL) and incubated for 24h. After incubation, the overnight grown cells were treated with MTT(5mg/mL) in PBS and incubated for 3h. The formazan crystals formed were dissolved using DMSO (100 μl) solution. The absorbance was measured at 570 nm using a microtiter plate.
Staining of autolysosomes by acridine orange
The colo205 cells were seeded in 24 well plates at 5 × 103 cells/well treated with LPS(1mg/mL) and incubated for 24 h. After 24 h the LPS-induced cells were washed with 1x PBS with different concentrations of peptide ranging from (25-125 µg/mL) and cultured for 24 h growth period. Then the cells were stained with acridine orange dye for 30 min, which was then examined using a fluorescence microscope [18].
Toxicity evaluation of peptide in-vivo
The experimental BALB/c mice about 8 weeks of 20-25g were purchased from TANUVAS with IAEC Approval No. SAF/200711/04. The acute toxicity study was conducted to determine the adverse effects of the peptide according to the (OECD) guideline 420 [19]. The mice in Group II-VI (n=5) received peptide concentrations of (50,100,150,200 & 250) mg/kg BW respectively, while the control group (n = 5) received no treatment. The experimental animals were maintained under observation for 14 days. On day 15th, the mice were anesthetized using ketamine and xylazine and sacrificed using cervical dislocation. For histopathological examination, the tissues were fixed with 10% formalin for 6-12 h.
In designing the experiments for the acute toxicity study, the animals were divided into six groups with five animals each. Group I (control group): received water at an oral dose for 14 days. Group II: Animals received peptide at an oral dose of 50 mg/kg BW. Group III: Animals received peptide at an oral dose at dose 100 mg/kg BW. Group IV: Animals received peptide at an oral dose of dose 150 mg/kg BW. Group V: Animals received peptide at an oral dose of 200 mg/kg BW. Group VI: Animals received peptide at an oral dose of 250 mg/kg BW. All dose ranges were studied for 14 days.
Statistical analysis
Statistical analysis was performed using Graph-Pad Prism 6 software. The data obtained for the study in finding differences among the control and treatment groups were analyzed using one-way ANOVA analysis. All data were expressed as mean ± SD. Statistical significance **** was set at P < 0.0005.
RESULTS
Characterization of the peptide from Momordica dioica using HPLC-MS and MALDI-TOF analysis
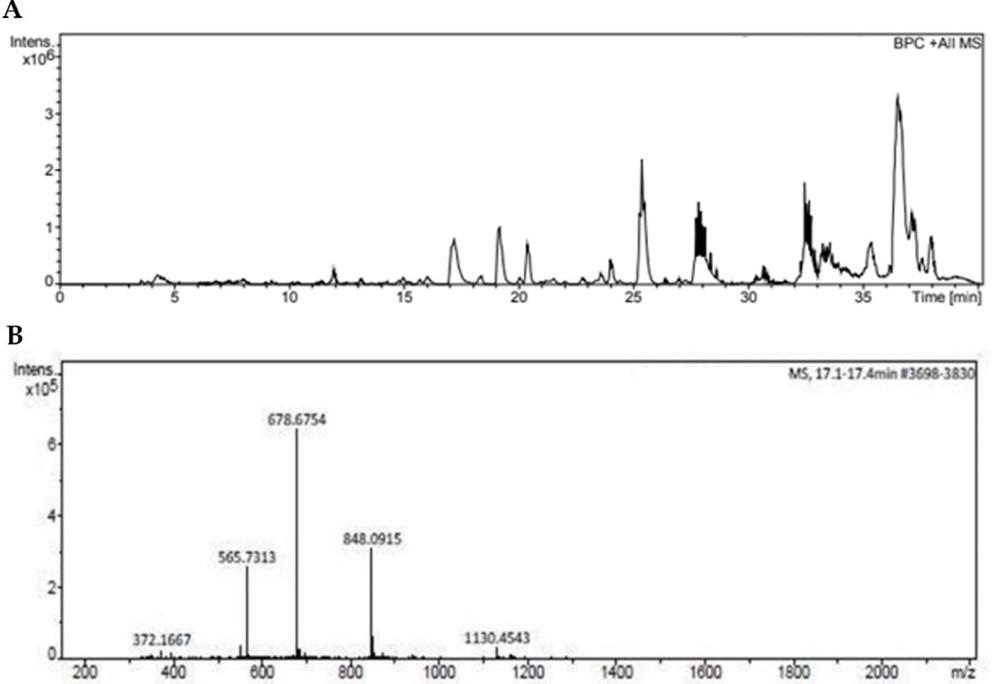
The HPLC-MS analysis of peptide was shown in (Figure 1A) which represents the peaks obtained after 15 min indicating late eluting hydrophobic peptide at 17 min. The peaks in the chromatogram (Figure 1B) represent the molecular mass analysis of the peptide which was found to be 678.67 Da which confirms the tryptic digestion of peptide.
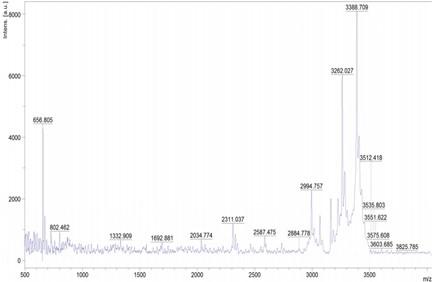
The peptide from the hydrolyzed sample was identified using MALDI-TOF, and the results are shown in (Figure 2) which indicates peaks obtained are found in the range of 3388.67 Da.
Anti-proliferative effect of peptide from Momordica dioica on Colo-205 cells
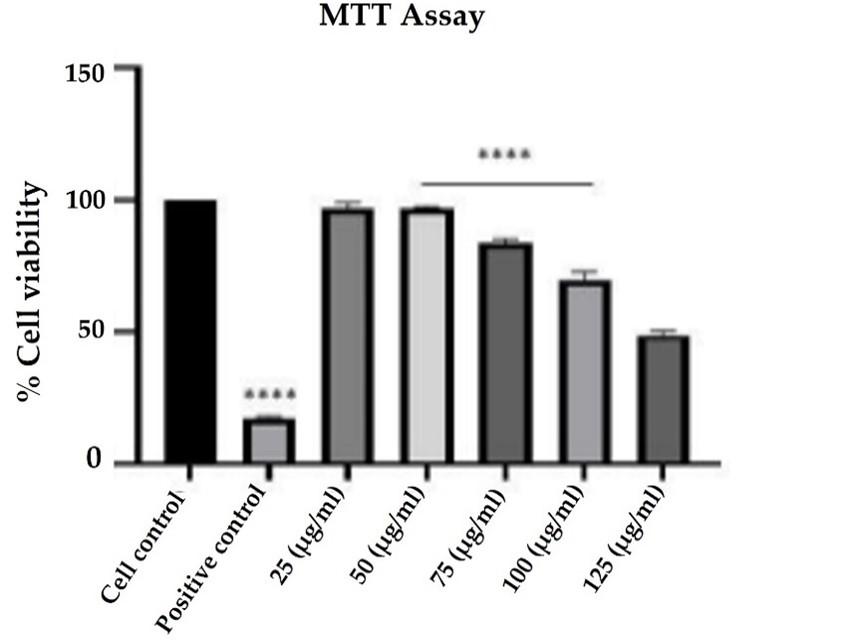
The MTT assay was performed to check the cell viability of the cells before and after drug treatment strategies. The peptide from Momordica dioica significantly inhibited the viability of the cells in a dose-dependent manner. When compared to control cells, a substantial decrease in cell viability was observed with the increasing concentrations of 25 to 125 µg/mL of peptide with an IC50 value of 100 µg/mL (Figure 3).
Autophagy induction in peptide-treated Colo-205 cells
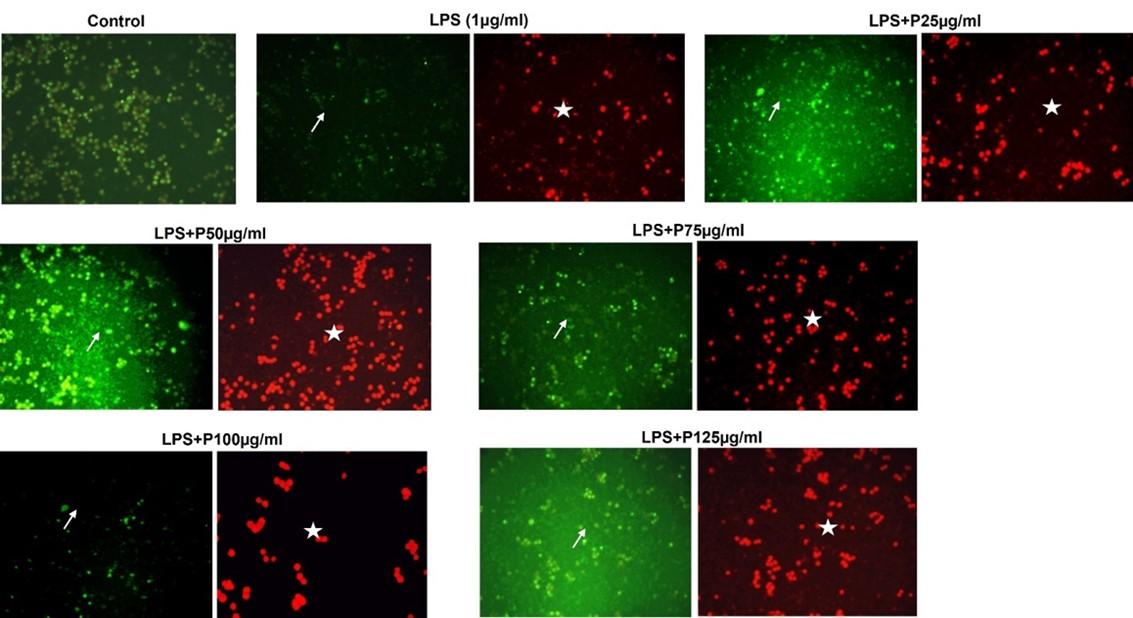
Colo 205 cells were treated with 25-125 µg/mL of peptide for 24 h, and the results shown in Figure 4 indicated that the cells treated at different concentrations showed significant morphological autophagic changes. The nuclei are shown in green fluorescence, whereas the autolysosomes are shown in red fluorescence. The colo205 cells treated with 100 µg/mL represent a significant decrease in the green nuclei cells compared to control cells indicating higher autophagy of the cancer cells.
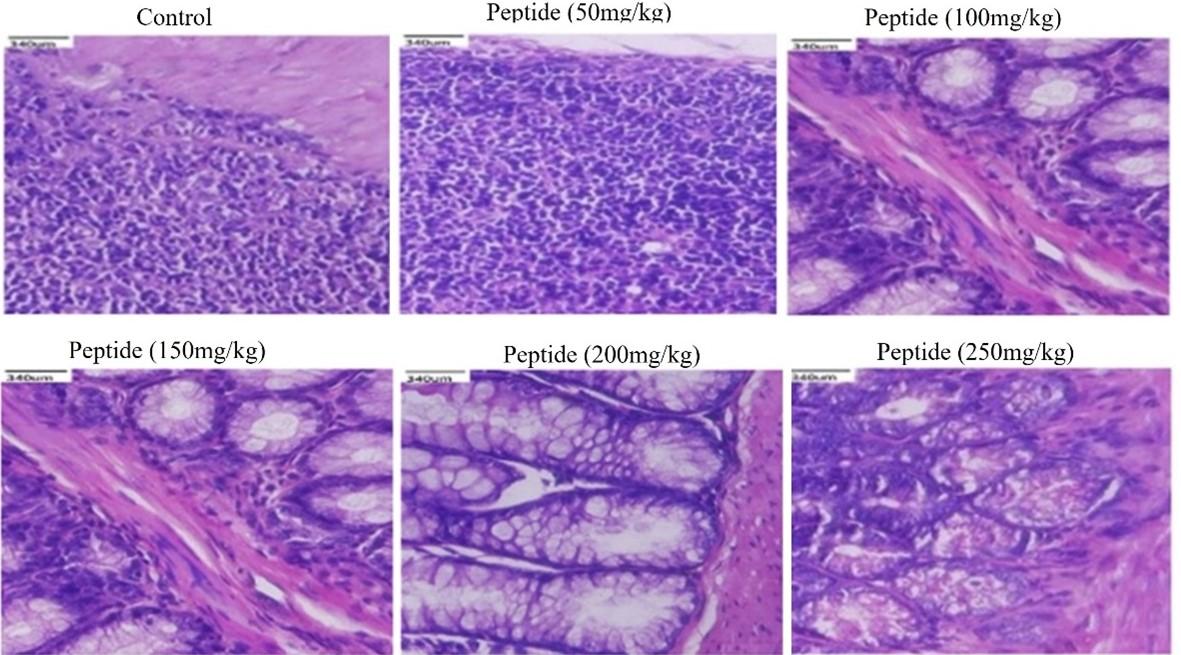
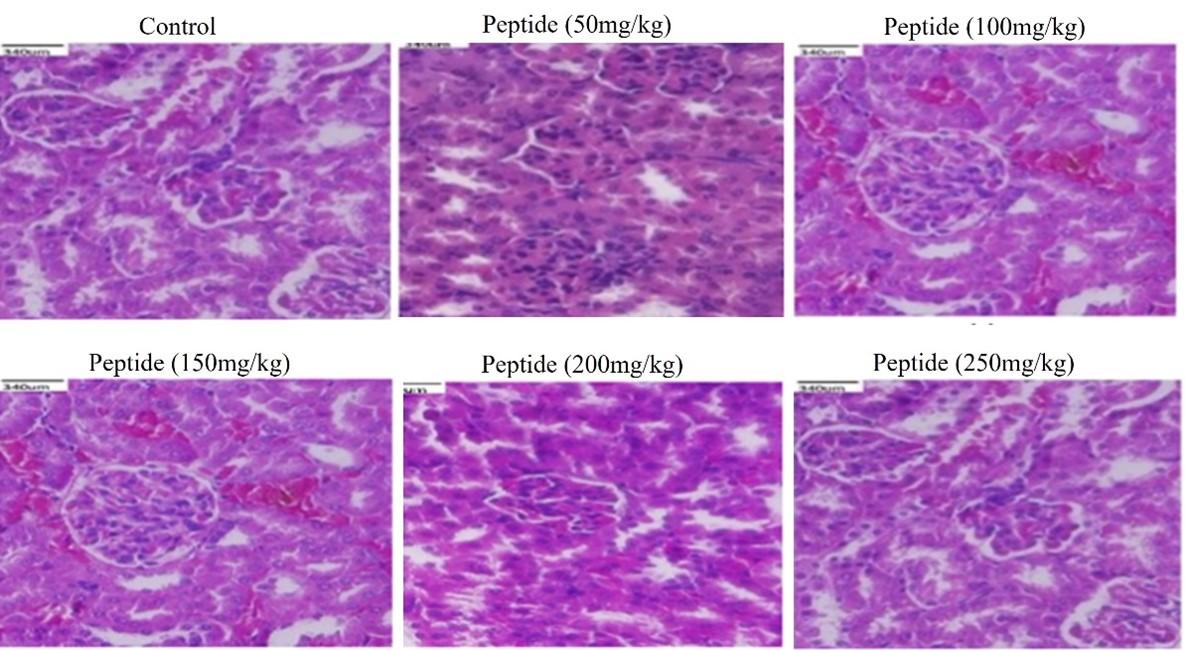
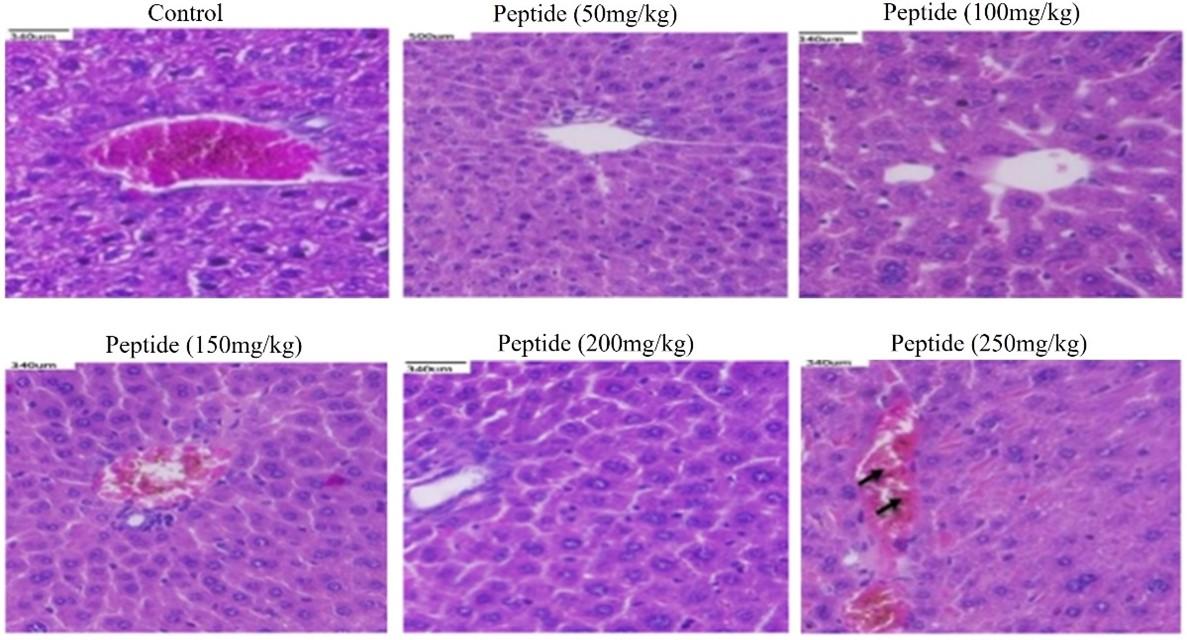
Histological examination of in-vivo samples treated with peptide
Histological evaluations of the kidneys, liver, and colon of mice showed no changes when compared to the control. The peptide treated groups with 50-200mg/kg respectively showed no signs of toxicity or injury in colon (Figure 5), kidney (Figure 6), and liver (Figure 7) compared to the control group. However, the liver tissue with peptide treatment (250mg/kg), examined mild periportal inflammation (Figure 7). Table 1 revealed no significant differences for behavioral changes between the peptide treatment group (II-V) while a significant difference in body weight was observed for the treatment group (VI) compared to control groups.
Table 1. Behavioral responses and general parameters observed in mice treated with different doses of peptide.
DISCUSSION
The current study demonstrated the effect of the isolated peptide dialysate on the cell viability of Colo205 cells induced with Lipopolysaccharide.LC-MS analysis of our peptide was found to be in the range of 1000Da. Cao et al., [20] studied the trypsin hydrolysis of enzyme and obtained four peaks in the range of 3KDa with its structural characterization from the Momordica species. This study’s results are in accordance with a previous study that discovered that peptide of 2000 Da exhibited a greater anti-inflammatory effect in protein hydrolysates made from a combination of proteases [21]. Previously reported by Xiang et.al., [22] showed hydrolysates of rice drew showed bioactive peptide with a less molecular weight greater than 1KDa.
MALDI-TOF analysis results showed bioactive peptide with 500-4000Da. Similar peaks with masses were found to be 1246.6 Da, 1723.8 Da, 1858.6 Da, 822.4 Da, 1435.7 Da, and 3279.2 Da after chymotryptic proteolysis using the Citrullus colocynthis a Cucurbitaceae plant family [23]. A similar study showed the MALDI-TOF of hydrolysates from Tinospora cardifolia produced by gastric enzyme cleavage with a mass of 1450.71 and 1023.51Da showed decreased level of oxidative stress parameters and inflammatory activity [24]. Peptide from egg white using pepsin hydrolysate showed the molecular weights of 3KDa which were shown to improve the antioxidant activity [25].
The MTT assay analyzed the cell viability of colo205 cells treated with an increasing dose of peptide dialysate concentration in which 75μg/mL was the concentration at which viability is maintained at 70%. A previous study reported a dose-dependent reduction in viability of leukemic cells of Molt-4 treated with hydrolysate of sesame seeds by pepsin enzyme [26]. Similar studies were carried out for the anticancer property of Morinda Pubescens peptide against human cancer cells A459 [27].
The autolysosome formation in the cells is a marker of autophagy stained with acridine orange dye [28]. The study reveals that the peptide in the increasing concentration shows autophagic cell death of colo205 cells at 75μg/mL. The morphological features of autophagy cells in earlier studies using PC-3 cells treated with oligopeptide QPK [29] and HeLa cells treated with a hexapeptide with sequence Phe-Ile-Met-Gly-Pro-Tyr extracted from Raja porosa [30,31].
The in vivo acute toxicity and the behavioral changes were studied with the increasing dose (50-250mg/kg BW) of the peptide for the BALB/c mice. In this study, we observed a change in the morphology of the liver with the treatment of 250 mg/kg of peptide compared to the control group that received only water. This was in line with the study by Amare [32] who showed the presence of morphological change between the control group and mice treated with 200 mg/kg. A study similar to the current study showed that whey protein hydrolysate (200-600 mg/kg) in mice showed no signs of structural changes in the organ after histopathological examination [33].
CONCLUSION
In this study, the peptide of molecular mass 3388.7Da showed no signs of symptoms and toxicity in in-vivo study, which are evident from the microscopic observation of the tissues. Further, the peptide -induced cell death by showing the presence of acidic vacuolar organelles, and autolysosomes indicating autophagy induction. Therefore, the purified Peptide was shown to decrease the inflammation process through the upregulation of autophagic proteins in colon cancer cells.
ACKNOWLEDGEMENT
None.
AUTHOR CONTRIBUTIONS
RS designed the outline of the study and JS drafted the manuscript. JS performed the experiments and analyzed the data. JS wrote the initial draft of the manuscript. RS reviewed the scientific contents described in the manuscript. RS read and approved the final submitted version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Alimentary pharmacology & therapeutics 2006; 23:1097-1104.
- [2]Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. Journal of immunology research 2019; 2019:1-16.
- [3]Vilela EG, da Gama Torres HO, Martins FP, de Abreu Ferrari MD, Andrade MM, da Cunha AS. Evaluation of inflammatory activity in Crohn’s disease and ulcerative colitis. World journal of gastroenterology: WJG 2012; 18:872-881.
- [4]Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. Journal of inflammation research 2014;7: 113-120.
- [5]van Leuven SI, Hezemans R, Levels JH, Snoek S, Stokkers PC, Hovingh GK, Kastelein JJ, Stroes ES, de Groot E, Hommes DW. Enhanced atherogenesis and altered high density lipoprotein in patients with Crohn’s disease. Journal of lipid research 2007; 48:2640-2646.
- [6]azou A, Ikonomidis I, Bartekova M, Benedek T, Makavos G, Palioura D, Cabrera Fuentes H, Andreadou I. Chronic inflammatory diseases, myocardial function and cardioprotection. British Journal of Pharmacology 2020; 177:5357-5374.
- [7]Mato AR, Hill BT, Lamanna N, Barr PM, Ujjani CS, Brander DM, Howlett C, Skarbnik AP, Cheson BD, Zent CS, Pu JJ. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Annals of Oncology 2017;28:1050-1056.
- [8]Anderson P, Delgado M. Endogenous anti-inflammatory neuropeptide and pro‐resolving lipid mediators: a new therapeutic approach for immune disorders. Journal of cellular and molecular medicine 2008; 12:1830-1847.
- [9]Chakrabarti S, Guha S, Majumder K. Food-derived bioactive peptide in human health: Challenges and opportunities. Nutrients 2018;10:1-17.
- [10]Netea-Maier RT, Plantinga TS, van de Veerdonk FL, Smit JW, Netea MG. Modulation of inflammation by autophagy: consequences for human disease. Autophagy 2016;12:245-260.
- [11]Chew TS, O’Shea NR, Sewell GW, Oehlers SH, Mulvey CM, Crosier PS, Godovac-Zimmermann J, Bloom SL, Smith AM, Segal AW. Optineurin deficiency in mice contributes to impaired cytokine secretion and neutrophil recruitment in bacteria-driven colitis, Disease models & mechanisms 2015;8:817-829.
- [12]Varghese J, Gajbhiye A. Purification and characterization of trypsin inhibitor protein from seeds of Momordica dioica. Asian Journal of Pharmaceutical Clinical Research 2016; 3:335- 339.
- [13]Jabeen U, Khanum A. Isolation and characterization of potential food preservative peptide from Momordica charantia L. Arabian Journal of Chemistry 2017;10: S3982-S3989.
- [14]Varghese J, Gajbhiye, A., Characterization of Protein from Momordica dioica Seeds for Trypsin Inhibitory Activity and its Mass Spectrometric Analysis. journal de afrikana 2016;3: 214-224.
- [15]Mikulášek K, Konečná H, Potěšil D, Holánková R, Havliš J, Zdráhal Z. SP3 Protocol for Proteomic Plant Sample Preparation Prior LC-MS/MS. Frontiers in plant science 2021;12:1-10.
- [16]Livet S, Worbs S, Volland H, Simon S, Dorner MB, Fenaille F, Dorner BG, Becher F. Development and evaluation of an immuno-MALDI-TOF mass spectrometry approach for quantification of the abrin toxin in complex food matrices. Toxins 2021; 13:52-60.
- [17]Kim HJ, Park CG, Varghese R, Lee JY, Kim Y, Sung GH. In-vitro antioxidative, anti-inflammatory properties of Aurea helianthus leaf extract a Korean traditional medicinal plant. Saudi journal of biological sciences 2017; 24:1943-1947.
- [18]Hussain H, Ahmad S, Abd. Razak MF, Wan Mohamud WN, Bakar J, Ghazali HM. Determination of cell viability using acridine orange/propidium iodide dual-spectrofluorometric assay. Cogent Food & Agriculture 2019; 5:1-9.
- [19]Dash P, Rath G, Ghosh G. In Vivo Antioxidant Potential of Protein Hydrolysates of some Cucurbitaceae Seed. Journal of Drug Delivery and Therapeutics 2020;10(3):128-132
- [20]Cao H, Sethumadhavan K, Grimm CC, Ullah AH. Characterization of a soluble phosphatidic acid phosphatase in bitter melon (Momordica charantia). PloS one 2014; 9:1-10.
- [21]Ambigaipalan P, Al-Khalifa AS, Shahidi F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. Journal of Functional Foods 2015; 18:1125-1137.
- [22]Li X, Xiong H, Yang K, Peng D, Peng H, Zhao Q. Optimization of the biological processing of rice dregs into nutritional peptide with the aid of trypsin. Journal of Food Science and Technology 2012 ;49:537-546.
- [23]Shahin-Kaleybar B, Niazi A, Afsharifar A, Nematzadeh G, Yousefi R, Retzl B, Hellinger R, Muratspahić E, Gruber CW. Isolation of cysteine-rich peptide from Citrullus colocynthis. Biomolecules 2020; 10:1-17.
- [24]Pachaiappan R, Tamboli E, Acharya A, Su CH, Gopinath SC, Chen Y, Velusamy P. Separation and identification of bioactive peptide from stem of Tinospora cordifolia (Willd.) Miers. PloS one 2018; 13:1-10.
- [25]Dávalos A, Miguel M, Bartolomé B, Lópezfandiño R. Antioxidant activity of peptide derived from egg white proteins by enzymatic hydrolysis. Journal of Food Protocol 2004; 67:1939–1944.
- [26]Deesrisak K, Yingchutrakul Y, Krobthong S, Roytrakul S, Chatupheeraphat C, Subkorn P, Anurathapan U, Tanyong D. Bioactive peptide isolated from sesame seeds inhibits cell proliferation and induces apoptosis and autophagy in leukemic cells. EXCLI journal 2021; 20:709- 716.
- [27]Thomas AS, Saravanakumar R, Gupta PV. Evaluation of cytotoxic activity of protein extracts from the leaves of Morinda pubescens on human cancer cell lines. Revista Brasileira de Farmacognosia 2017; 27:99-104.
- [28]de Francisco L, Pinto D, Rosseto H, Toledo L, Santos R, Tobaldini-Valério F, Svidzinski T, Bruschi M, Sarmento B, Oliveira MB, Rodrigues F. Evaluation of radical scavenging activity, intestinal cell viability and antifungal activity of Brazilian propolis by-product. Food Research International 2018; 105:537- 547.
- [29]Huang F, Yang ZS, Yu D, Wang J, Li R, Ding G. Sepia ink oligopeptide induces apoptosis in prostate cancer cell lines via caspase-3 activation and elevation of Bax/Bcl-2 ratio. Marine Drug 2012; 10:2153–2165
- [30]Pan X, Zhao YQ, Hu FY, Chi CF, Wang B. Anticancer activity of a hexapeptide from Skate (Raja porosa) cartilage protein hydrolysate in HeLa Cells. Marine Drugs 2016; 14:153-162.
- [31]Xu G, Yan X, Hu Z, Zheng L, Ding K. Glucocappasalin Induces G2/M-Phase Arrest, Apoptosis, and Autophagy Pathways by Targeting CDK1 and PLK1 in Cervical Carcinoma Cells. Frontiers in pharmacology 2012;12: 1-15.
- [32]Abba S, Omotoso OD, and Joseph MI. Hemorrhagic centrolobar necrosis and cytoplasmic vacuolation of the hepatocytes in Syzygium guineense chronic treated mice. International Journal of Anatomy and Applied Physiology 2018;4: 99–102.
- [33]Hussein FA, Chay SY, Ghanisma SBM, Zarei M, Auwal SM. et al. Toxicity study and blood pressure–lowering efficacy of whey protein concentrate hydrolysate in rat models, plus peptide characterization. Journal of dairy science 2020;103: 2053-2064.