Accessing the prevalence of cancer biomarkers in suspected patients from northeastern part of Bangladesh
Abstract
Early detection and/or characterization of cancer-associated biomarkers have revolutionized the personalized treatment approach of cancer to date. Therefore, this study was designed to access the prevalence of suspected cancer biomarkers from northeastern part of Bangladesh. A total of 892 patients’ data were analyzed in a cross-sectional manner and the collection period was 2017 to 2019. An ELISA-based chemiluminescent microparticle immune assay was used to detect serum cancer biomarkers. An unpaired t-test was used to compare the independent variables. Data were clustered into four categories: group1 (10-17 yr, adolescent), group2 (18 to 35 yr, young adult), group3 (36 to 55 yr, middle), and group4 (>55 yr, older adult). Serum prostate-specific antigen (PSA) level was higher in group4 than group3 (8.422 ± 1.423 vs 3.884 ±1.15 ng/ml). Cancer antigen (CA)-125 is significantly higher in females with age > 35 yr (older and middle) than at age <35 yr (young adult and adolescent). No significant difference in AFP was found among the four age groups while sexual disparity has shown a statistically significant difference. Both males and females from group4 were showing a higher expression level of carcinoembryonic antigen (CEA) (96.94 ± 54.02 and 48.23 ± 25.45 ng/ml, respectively) though gender-wise test frequency was same. CA19-9 was high in group4 whereas no correlation was found between the gender variation. Thus, the study has shown that level of PSA, CA-125, CEA and CA19-9 were upregulated in older peoples, while AFP was higher in male than female.
INTRODUCTION
The sharp increase in cancer incidents is one of the important causes of morbidity and mortality, around the world, and so does Bangladesh. Alarmingly, it is predicted that the incidence of cancer cases will rise from 12.7 to 21.4 million from the year 2008 to 2030 [1]. In addition, cancer was ranked sixth in 1990 for causing global deaths, has shifted to second place in 2017, and in Bangladesh, it is the 6th leading cause of death, while 66% of cancer patients within the age range of 30 to 65 years, which, however, the main workforce population in any country [1, 2]. The risk factors of cancer are almost similar globally, though the prevalence of different factors varies by region and country [3, 4]. Despite the risk factors, it is essential to perform early detection, which is the paramount priority for proper management of cancer treatment and to provide a quality life span for the patients.
Several approaches have been used for cancer diagnosis and prognosis, for example genetic, epigenetic, proteomic, and imaging to identify biomarkers for the respective cancer. Though, at some point for cancer patient management a number of gene and protein based biomarkers have already been used; including, prostate-specific antigen (PSA; prostate cancer) [5], carbohydrate antigen 125 (CA-125; ovarian cancer) [6], carcinoma antigen 15-3 (CA15-3; breast cancer) [7] alpha-fetoprotein (AFP; liver cancer) [8], epidermal growth factor receptor (EGFR; non-small-cell lung carcinoma) [9], cancer antigen 19-9 (CA19-9; pancreatic cancer) [10], carcinoembryonic antigen (CEA; colorectal cancer) [11], type III receptor tyrosine kinase (KIT; gastrointestinal stromal tumour) [12], S-100 protein (S100; melanoma) [13] and many others.
It is apparent, to understand the biomarker of targeted cancer which is the key molecules that indicate normal or abnormal processes taking place in our physiological system. An underlying condition, a cancer biomarker will eventually guide the physician to set a treatment goal as each biomarker work within the body and responds differently to treatments regime. Cancer is still an incurable disease, which may be due to late-stage detection while early-stage detection has become a long-standing concern in the scientific community to date. Moreover, the dynamic nature of cancer biomarkers that occur at a certain population level and along with clinical data is essential to assess the prognosis of cancer.
The possible scenario of various cancer biomarkers from the northeastern part of (Sylhet division) Bangladesh is unknown due to the scarcity of published data, such as PSA, CA125, CA15-3, AFP, CA19-9, and CEA. Moreover, how and to what extent the expression of those cancer biomarkers is associated in the context of age and sex to the local people in Sylhet is yet to be determined. Since, the cancer-related death rate varies among sex, age, and regional differences [14-16].
MATERIALS AND METHODS
Study design and data collection
A total of 892 patients’ clinical data were collected from the year 2017 to 2019 from different medical diagnostics centres in the northeastern part (i.e., Sylhet city) of Bangladesh. This is a population-based cross-sectional study, where the data of various cancer biomarkers (e.g., PSA, CA125, CA15-3, AFP, CEA, CA19-9) were collected, prescribed by the local medical doctor/physician on the suspect case from various diagnostic medical centre of Sylhet. Proper formalities and ethical issues were maintained to extract the clinical data (e.g., id, age, sex, date, and values of respective cancer biomarkers) from the medical centres. All the essential steps and methods used in this proposed study were approved by the internal ethics committee (Ref# BMB/EC/001/2017) at the department of Biochemistry and Molecular Biology, School of Life Sciences, Shahjalal University of Science and Technology (SUST), Bangladesh.
ELISA-based chemiluminescent microparticle immune assay
Chemiluminescent microparticle immune assay was used to detect the expression level of the respective biomarker of suspected cancer accordingly to manufacturer instruction (ARCHITECT i1000SR immunoassay analyzer, Abbott, USA). Patients whole blood was collected in serum separator tube (BD Vacutainer® SST™, Georgia, United States) and allowed for 30 minutes at room temperature for clotting, and serum was separated by centrifuging at 1000-1500 x g for 15 minutes at room temperature. For each sample around 100-150µl serum was used and the accuracy of each/daily run was confirmed through standard calibration protocol. In brief, this assay is an automated two-step immunoassay to detect the presence of respective biomarkers in human serum, plasma, and amniotic fluid, using chemiluminescent microparticle immunoassay (CMIA) technology. Sample, assay diluent, and anti-biomarker coated paramagnetic microparticles are combined in the first step. The target biomarker/antigen present in the sample binds to the first anti-biomarker-coated microparticles followed by washing, then the second acridinium-labelled anti-biomarker as conjugate is added in the second step. Pre-trigger (1.32% hydrogen peroxide, w/v) and trigger (0.35N sodium hydroxide) solutions are then added to the reaction mixture. The resulting chemiluminescent reaction (correlate with the amount of biomarker present in the sample) is measured as relative light units (RLUs) by the ARCHITECT immune assay optical system. The reference number of ARCHITECT reagent kits that were used are 7K71, 2K45, 2K44, 7K67, 7K68, and 2K91 for PSA, CA-125, CA15-3, AFP, CEA, and CA19-9, respectively (Abbott Laboratories, Abbott Park, Illinois, USA).
Clinical data analysis
Patients were categorized into various age groups which were broadly defined, such as group1 (10-17 years), group2 (18 to 35 years), group3 (36 to 55 years), and group4 (>55 years and older) for clinical data analysis [17]. Sex-related vulnerability towards cancer vulnerability was also analysed.
Statistical analysis
All the collected data sets were evaluated with a multivariate statistical package, GraphPad Prism 7 software, (San Diego, CA, USA) and excel. A p-value <0.05 indicated by asterisk (significant), otherwise p value > 0.05 indicated by ns (not significant). An unpaired t-test or student’s t-test (two-tailed) was performed to assess the differences between the independent variables.
RESULTS
Characteristic features of the studied population
All the possible clinical data sets were collected over two the year of 2017-2019, based on the prescribed/suspected cancer by the local doctor/physician and those are, prostate-specific antigen (PSA), carbohydrate antigen 125 (CA-125), carcinoma antigen 15-3 (CA15-3), alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9) for prostate, ovarian, breast, liver, colorectal and pancreatic cancer, respectively. A total population, n=892 was analyzed to see the prevalence of cancer biomarkers in this local community based on age and sex variation of the individual. Among the six biomarkers, PSA is a male-specific marker [5] while CA-125 [6] and CA15-3 [7] refer to a female-specific marker. The rest of the three biomarkers are for both males and females. Moreover, the clinical data were categorized into four age groups (e.g., group1, 2, 3 and 4) that covered adolescents, adulthood, middle age, and older adult to see the cancer biomarkers distribution among the various aged group in both males and females. Our analysis did not account for the reference values for any biomarker being positive or negative for specific cancer, rather all the available values were used to get the overall scenario to set the sample selection criteria. Male and female participants were 52% and 48%, respectively. Among prescribe patients, prostate and ovarian cancer biomarkers were higher, which are 31.05% and 18.95%, respectively. On the other hand, breast, liver, colorectal, and pancreatic are 3.25%, 18.83%, 16.82%, and 11.10%, respectively (Figure 1A-C).
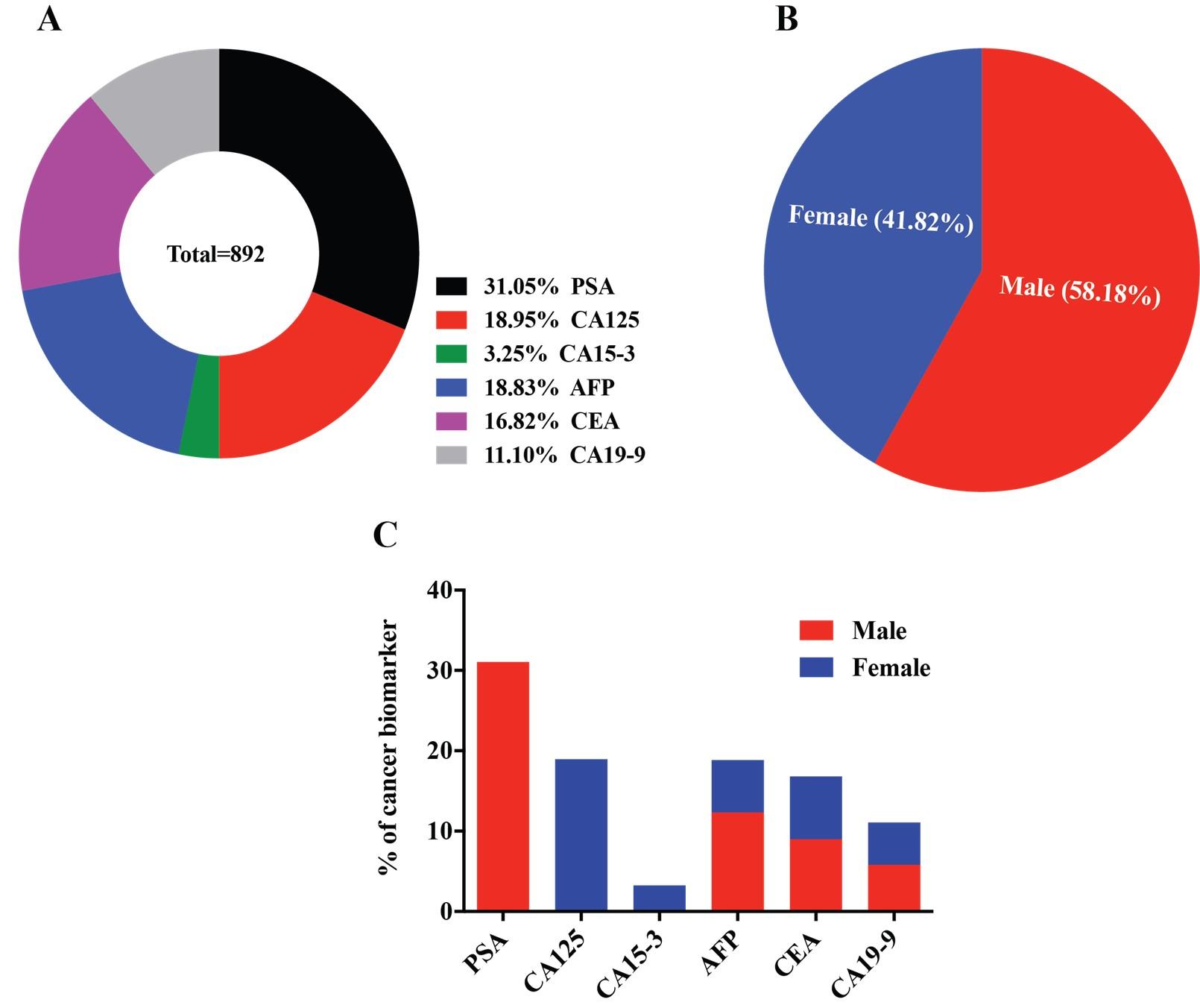
Gender associated cancer biomarkers
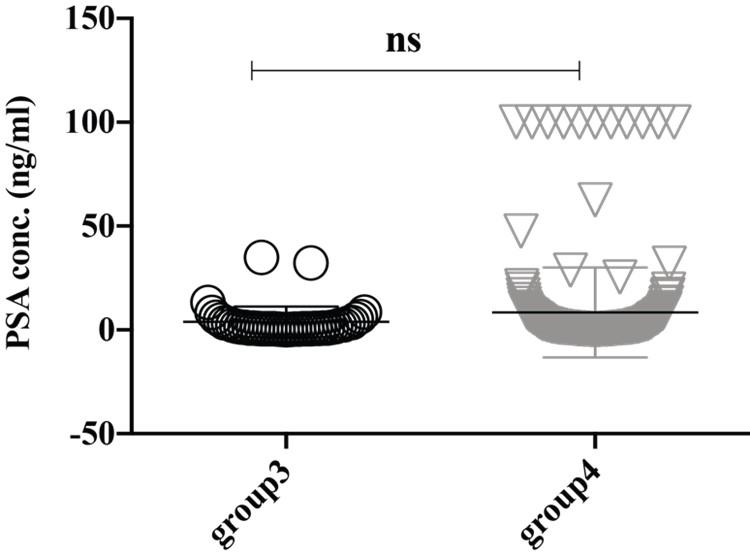
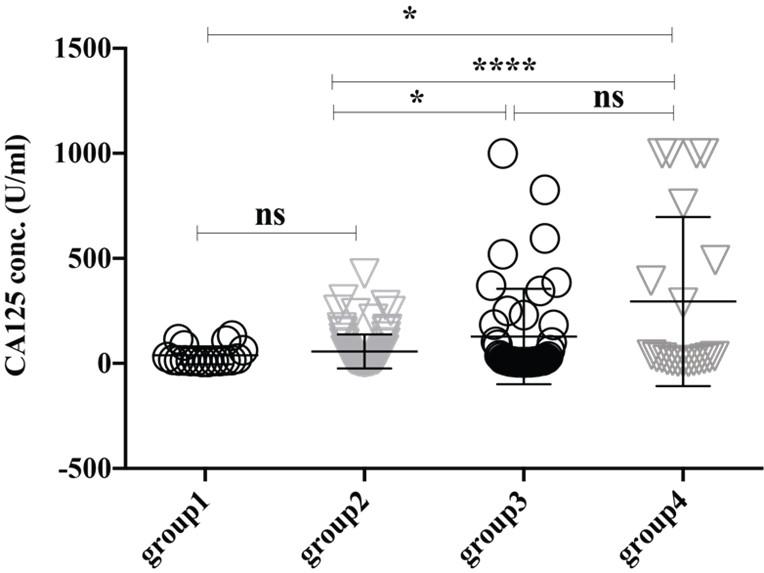
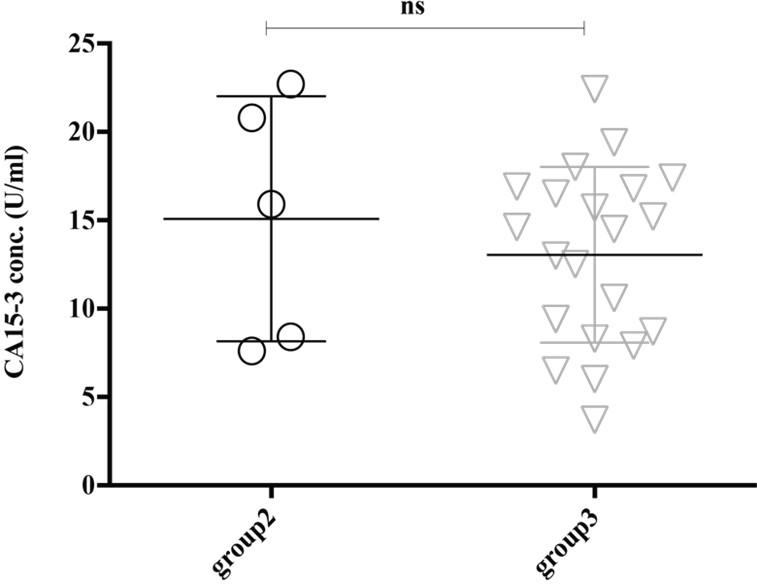
The expression level of serum PSA (ref value, 0-4 ng/ml) seems higher in the group4 (8.422 ± 1.423 ng/ml) though it didn’t reach significantly with the group3 (3.884 ±1.15 ng/ml) (Figure 2). Thus, our analysis also supports other studies that PSA is upregulated in older adult peoples and/or have a higher trend of developing prostate-related complication [18]. Female-specific cancer/tumor marker, CA-125 or MUC16 is used for the diagnosis and management of ovarian cancer. Significantly high expression of CA-125 was observed in the female in group4 (295.1 ± 87.75 U/ml) and group3 (127.7 ± 34.24 U/ml) than in group2 (57.11 ± 8.653 U/ml) and group1 (38.66 ± 9.95 U/ml), where the reference value is <35.00 U/ml (Figure 3). Therefore, this analysis indicates the striking rate of ovarian cancer incidence in this small locality of Bangladesh. Another female-specific cancer biomarker is CA15-3, used to monitor breast cancer treatment and disease recurrence and the reference range in serum is less than 30 U/mL. The expressional level of CA15-3 seems same between the group2 (15.08 ± 3.099 U/ml) and group3 (13.05 ± 1.086 U/ml) and which is not statistically significant (Figure 4).
Cancer biomarkers common for both gender
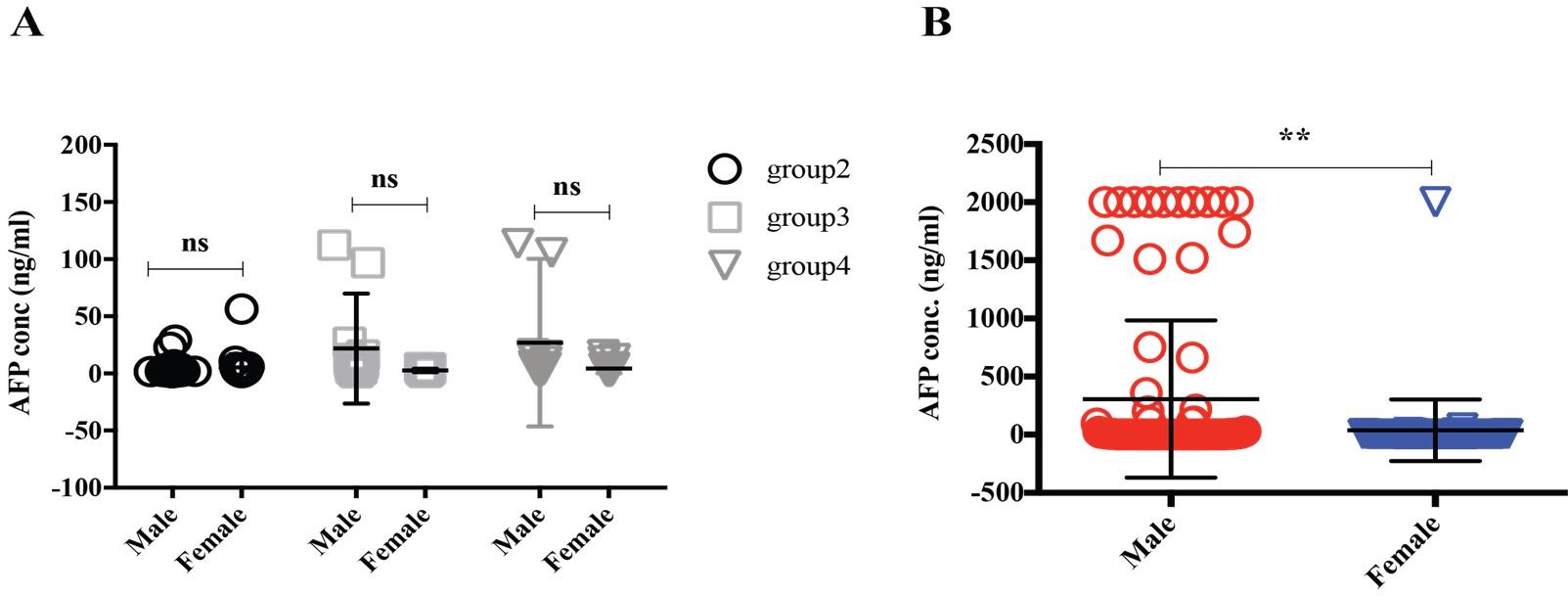
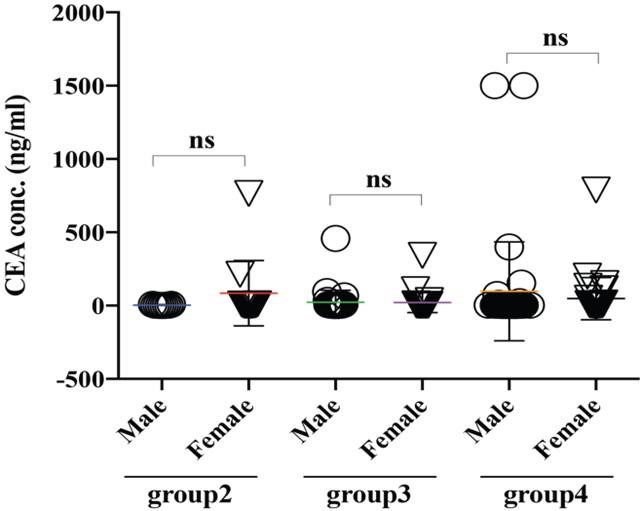
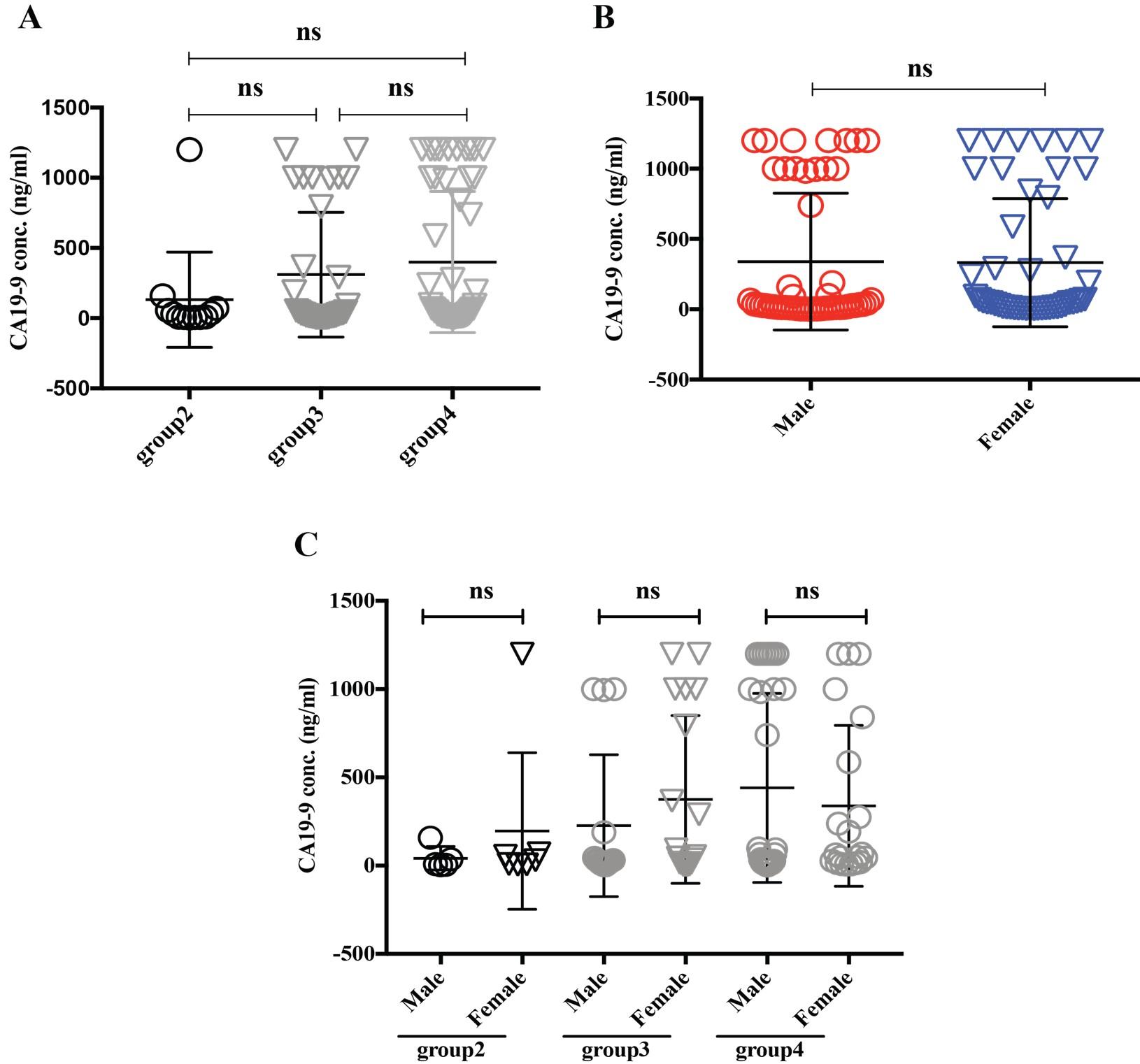
Alpha-feto protein (AFP) is accepted as a very useful serum marker for the detection of hepatocellular carcinoma [19]. Our analysis didn’t find any significant differences among the group2, 3 and 4 (Figure 5A), interestingly when we regroup the data sets based on sexual disparity, we have seen male patients (307.05 ng/ml) expressed a significantly higher level of AFP than female (39.31 ng/ml), suggesting male individual may be more vulnerable to develop a liver-related complication (Figure 5B). A carcinoembryonic antigen (CEA) test is used to help diagnose and manage the cancer of the large intestine and rectum. Generally, CEA is present at very low levels in the blood of healthy adults (2-4 ng/mL). Although the same frequency of CEA suspected in males and females was found in this study population (Figure 1B), which, however, also reflected among the various age groups between males and females (Figure 6). Besides, higher level of CEA (for male and female, are 96.94 ± 54.02 and 48.23 ± 25.45 ng/ml, respectively) was found in the older adult group4 patients irrespective of gender, which is in good agreement with other studies [2]. Cancer antigen 19-9 (CA19-9) is a protein that expresses on the surface of cells with pancreatic cancer [10]. In our data analysis, the CA19-9 expression level was high in group4 and group3 (399.5 ± 69.7 and 310 ± 76.17 ng/ml) concerning group2 (132.3 ± 97.92 ng/ml), though it didn’t reach statistically significant (Figure 7A). Further analysis also revealed CA19-9 expression profile was almost the same between male and female patients regardless of any age group (Figure 7B-C).
DISCUSSION
In this current study, we have characterized the available cancer biomarkers (e.g., PSA, CA125, CA15-3, AFP, CEA, and CA19-9) in the northeastern part of Bangladesh. Generally, suspicion of prostate and ovarian cancer in male and female were high in this region. Prostate cancer seems to have thrived in group4 (older adults) than in group3 (middle age) people supporting other studies that PSA upregulated or prostate cancer is a disease of the elderly [18]. Due to the lack of data from other aged groups, we are not sure about PSA level in younger peoples but it is already reported mortality of prostate cancer at a young age are almost negligible [18, 20]. Higher incidents of CA-125 in middle to older adult female from this small population is very alarming suggesting a family history of breast/ovarian cancer, post menopause, hormonal imbalance, overweight, obesity, and oral contraceptive are the few factors that may be associated with ovarian cancer in later life in female. A study in Sweden where 5550 women were enrolled for CA-125 screening and the author concluded that a higher level of CA-125 was found in women older than 50 years which is in good agreement with our study [21]. However, these finding needs to explore not only in other urban cities but also in other suburbs. In general, the higher the CA15-3 level the more advanced the breast cancer and the greater the tumor burden [22]. In addition, early detection of this biomarker increases the survival rate of patients from 80% to 92% whereas late or metastatic detection causes this rate down to 25% [23]. It is important to note that, our analysis may fail to get the real picture of the breast cancer scenario due to the small sample size (n=26 for CA15-3).
AFP is elevated in hepatocellular carcinoma (HCC) and early detection is highly desirable as patients with initial stages are often asymptomatic and frequently diagnosed at the late stage, when it is untreatable [24]. It has been reported that AFP has a significant correlation with the size of the tumor [25], while HCC affects men more commonly than women [26]. Remarkably, we have seen AFP levels significantly higher in male patients than in females. Irrespective of its a etiology, this sex disparity and AFP level of HCC are poorly understood and may be owing to multiple behavioral, and hormonal imbalances, and cancer biology/risk factors [26].
As an effective non-invasive blood test, serum CEA is routinely monitored in patients with colorectal cancer to assess the effectiveness of chemotherapy or radiation treatment [27, 28]. In our datasets, the older adult group4 was showing a higher expression level of CEA both in males and females, which supports the other study where higher CEA levels correlated with age, BMI, and poor control of diabetes [29], suggesting deteriorating health state of the elderly participants. Unfortunately, we couldn’t distinguish the CEA level based on smoker and non-smoker, as heavy smoker generally shows a higher level of serum CEA [30]. CA19-9 can be measured in blood and monitored to follow the course of pancreatic cancer and it became elevated in about 70% to 95% of people with advanced level [10] . Older patients have shown a higher level of CA19-9, while our analysis also revealed that there was no correlation between gender variation on its expression level in serum. Interestingly, CA19-9 and CEA have been used commonly for diagnosing pancreatic cancer [31], and in our datasets, these two biomarkers were higher in older group4 patients. However, due to a lack of information on CA19-9 levels from colorectal and CEA levels from pancreatic suspected patients, we couldn’t correlate any relation from our analysis regarding these two biomarkers.
However, our current study has some limitations and one of the major drawbacks was, whether the patients had under medication and the stage of cancer at the time of enrolment, which was not disclosed to us, this may be the policy of the medical center. The second limitation was, we couldn’t get datasets from all four groups of patients of the five biomarkers (e.g., PSA, CA15-3, AFP, CEA, and CA19-9), due to the nature of cancer and/or few participants from the respective group.
CONCLUSIONS
Retrieving the occurrence of cancer biomarkers in a local community not only reduces morbidity and mortality but also helps the doctor to set a treatment plan to save lives. Higher levels of serum PSA, CA-125, CEA, and CA19-9 were found in people of higher age. AFP was higher in males than females. Alarmingly, the CA-125 expression level was high in the female of age over 35 years, in this small population of Bangladesh. Therefore, it is an indication of the striking rate of ovarian cancer incidence may also prevail in other parts of Bangladesh. From our current preliminary study, it is imperative to explore this kind of cross-sectional study in other parts of Bangladesh, especially to see the real incident of PSA, CA-125, and AFP.
ACKNOWLEDGEMENT
We like to thank SUST Research Centre for their funding. We also like to thank doctors and technical staffs from different diagnostics and hospitals in Sylhet for their productive cooperation. We wish to thank all faculties, staff, and students from the department of Biochemistry and Molecular biology, SUST, Sylhet, for their generous help.
AUTHOR CONTRIBUTIONS
ZH, MH, and MWM were involved in the conception and design of the study. MH, MAC ZH, SS, and BC were involved in collecting data and analysis. ZH wrote the manuscript. MH, MWM, SS, and BC helped in revising the manuscript. MAH and MWM contributed to revising it critically for important intellectual content. ZH made the final approval of the version to be published.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Hussain SA, Sullivan R. Cancer control in Bangladesh. Jpn J Clin Oncol. 2013;43:1159-69.
- [2]Global Burden of Disease Cancer C, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-68.
- [3]Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716.
- [4]Morra A, Jung AY, Behrens S, Keeman R, Ahearn TU, Anton-Culver H, et al. Breast Cancer Risk Factors and Survival by Tumor Subtype: Pooled Analyses from the Breast Cancer Association Consortium. Cancer Epidemiol Biomarkers Prev. 2021;30:623-42.
- [5]Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239-46.
- [6]Scholler N, Urban N. CA125 in ovarian cancer. Biomark Med. 2007;1:513-23.
- [7]Terava J, Tiainen L, Lamminmaki U, Kellokumpu-Lehtinen PL, Pettersson K, Gidwani K. Lectin nanoparticle assays for detecting breast cancer-associated glycovariants of cancer antigen 15-3 (CA15-3) in human plasma. PLoS One. 2019;14:e0219480.
- [8]Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan D, et al. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One. 2020;15:e0228857.
- [9]Lamparella N, Barochia A, Almokadem S. Impact of genetic markers on treatment of non-small cell lung cancer. Adv Exp Med Biol. 2013;779:145-64.
- [10]Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-70.
- [11]Campos-da-Paz M, Dorea JG, Galdino AS, Lacava ZGM, de Fatima Menezes Almeida Santos M. Carcinoembryonic Antigen (CEA) and Hepatic Metastasis in Colorectal Cancer: Update on Biomarker for Clinical and Biotechnological Approaches. Recent Pat Biotechnol. 2018;12:269-79.
- [12]Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205-20.
- [13]Xiong TF, Pan FQ, Li D. Expression and clinical significance of S100 family genes in patients with melanoma. Melanoma Res. 2019;29:23-9.
- [14]Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2000;8:175-9.
- [15]Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-907.
- [16]Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885-92.
- [17]Petry NM. A Comparison of Young, Middle-Aged, and Older Adult Treatment-Seeking Pathological Gamblers. The Gerontologist. 2002;42:92-9.
- [18]Hussein S, Satturwar S, Van der Kwast T. Young-age prostate cancer. J Clin Pathol. 2015;68:511-5.
- [19]Seggie J, Gelfand M. Serum alpha-feto protein (alpha-FP) and hepatoma in Rhodesian Africans. Trans R Soc Trop Med Hyg. 1975;69:209-11.
- [20]Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate cancer in young men: an important clinical entity. Nat Rev Urol. 2014;11:317-23.
- [21]Einhorn N, Sjovall K, Knapp RC, Hall P, Scully RE, Bast RC, Jr., et al. Prospective evaluation of serum CA 125 levels for early detection of ovarian cancer. Obstet Gynecol. 1992;80:14-8.
- [22]Stieber P, Nagel D, Ritzke C, Rossler N, Kirsch CM, Eiermann W, et al. Significance of bone alkaline phosphatase, CA 15-3 and CEA in the detection of bone metastases during the follow-up of patients suffering from breast carcinoma. European journal of clinical chemistry and clinical biochemistry : journal of the Forum of European Clinical Chemistry Societies. 1992;30:809-14.
- [23]Safi F, Kohler I, Rottinger E, Beger H. The value of the tumor marker CA 15-3 in diagnosing and monitoring breast cancer. A comparative study with carcinoembryonic antigen. Cancer. 1991;68:574-82.
- [24]Mittal A, Sathian B, Chandrashekharan N, Farooqui SM, Hussain A. Diagnostic significance of alpha fetoprotein in carcinomas of liver and biliary tract – a comparative study from the western region of Nepal. Asian Pacific journal of cancer prevention : APJCP. 2011;12:3475-8.
- [25]Abbasi A, Bhutto AR, Butt N, Munir SM. Corelation of serum alpha fetoprotein and tumor size in hepatocellular carcinoma. J Pak Med Assoc. 2012;62:33-6.
- [26]McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-38.
- [27]Knychalski B, Lukienczuk T. The evaluation of diagnostic value of the tumor markers: CCSA-2 and CEA in colorectal cancer. Pol Przegl Chir. 2012;84:86-92.
- [28]Gobbi PG, Valentino F, Berardi E, Tronconi C, Brugnatelli S, Luinetti O, et al. New insights into the role of age and carcinoembryonic antigen in the prognosis of colorectal cancer. Br J Cancer. 2008;98:328-34.
- [29]Liu XY, Jin C, Zhou Y. High Prevalence of Abnormal Carcinoembryonic Antigen in Diabetic Inpatients with Poor Glycemic Control. Diabetes Metab Syndr Obes. 2022;15:2345-52.
- [30]Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Effect of histologic type and smoking status on interpretation of serum carcinoembryonic antigen value in non-small cell lung carcinoma. Ann Thorac Surg. 2004;78:1004-9; discussion 9-10.
- [31]Wu L, Huang P, Wang F, Li D, Xie E, Zhang Y, et al. Relationship between serum CA19-9 and CEA levels and prognosis of pancreatic cancer. Ann Transl Med. 2015;3:328.