Restoration of hepatorenal dysfunction and injury by zinc and folic acid combination in bisphenol A-intoxicated mice
Abstract
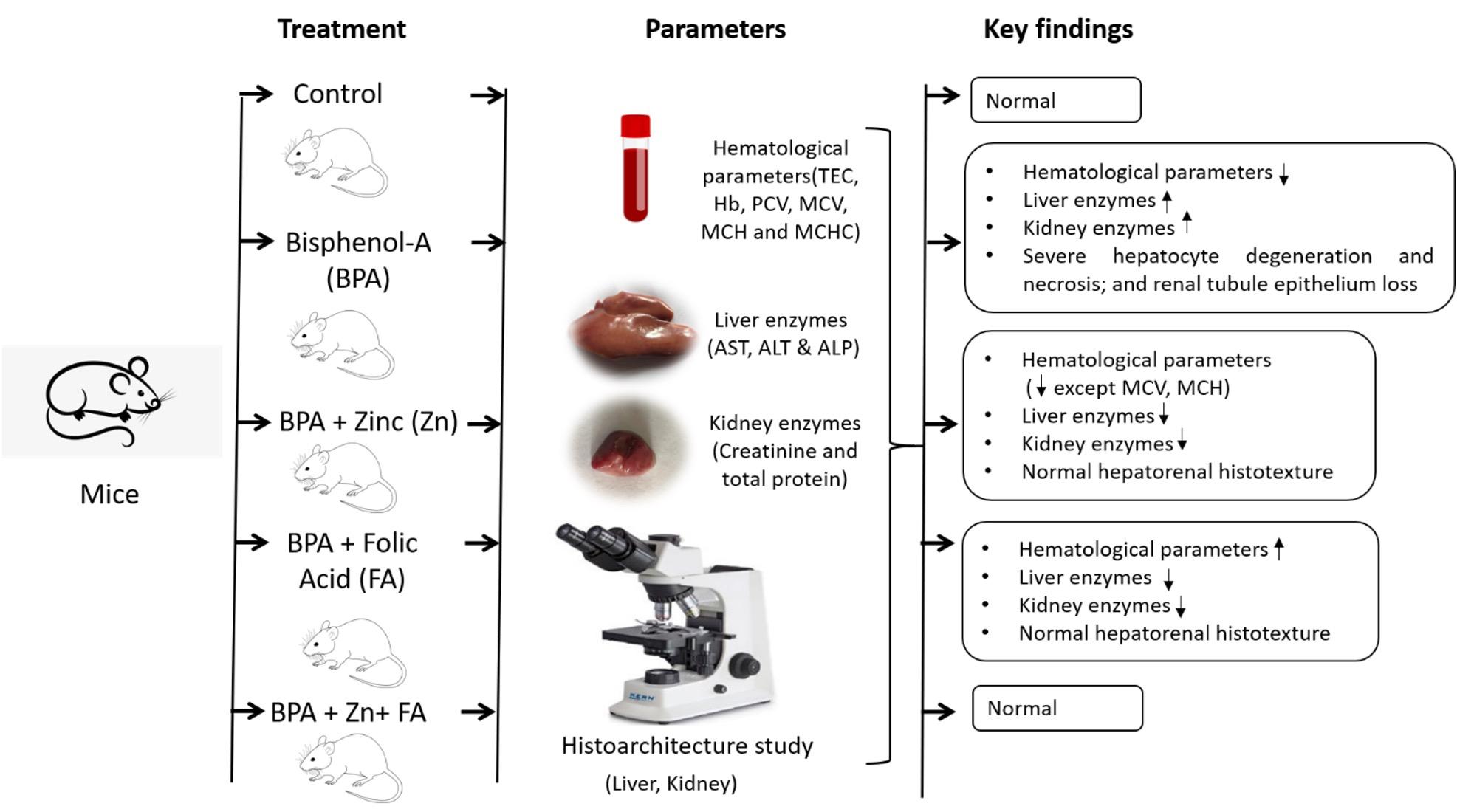
Bisphenol A (BPA) is a potentially hazardous substance and is extensively used in manufacturing industries as a plasticizing agent. The current research intended to determine the revival actions of zinc (Zn) and folic acid (FA) on hematological parameters and hepatorenal function in BPA-exposed male albino mice. A total of 75 adult male mice, aged 25 to 28 days, were split into 5 groups. Group A (control group) received a normal diet, while a daily dosage of 50 mg BPA/kg body weight (BW) in the diet was provided to groups B–E. Groups C, D, and E received daily supplementation of 10 mg Zn/kg BW, 3 mg FA/kg BW, or both in the feed. After 12 weeks of treatment, serum was prepared by collecting blood, and the kidney and liver were taken for a histotexture study. The results demonstrated that hematological values dropped significantly (p< 0.05) in the BPA-treated group and increased following Zn and FA supplementations, while no significant alterations in erythrocyte indices were observed among control and treated groups. The BPA group used to have significantly (p< 0.05) higher liver and kidney biomarkers levels, which were restored by Zn and FA. The effects of BPA administration included severe hepatocyte degeneration and necrosis, as well as renal tubule epithelium loss, all of which were improved by Zn and FA. Hence, the findings suggest that combination of Zn and FA could be potential preventative agents against BPA-induced toxicity.
INTRODUCTION
Bisphenol A (BPA) is an industrial chemical commonly used in manufacturing paper, food, consumer goods, beverage containers, and various industrial components [1]. Humans and animals are increasingly exposed to BPA because of leaching out of polymers and entering food and water bodies [2]. BPA is also reported in both solid as well as liquid forms of canned foods, with the solid fractions containing more than the liquid fractions [3]. Reduced hemoglobin concentrations are thought to be caused by decreased erythropoietin production due to BPA’s increased estrogenic activity or increased red blood cell destruction [4]. Acute BPA exposure significantly reduced hemoglobin content, red blood cell count, and PCV values [5]. Rats with oral BPA exposure for four weeks have considerably increased ALT and AST levels [6]. The hepatic system is a crucial organ for the breakdown of BPA detoxification. BPA induces hepatotoxicity, which results in hepatocyte death [7]. In the livers of rodents, mice, and humans, BPA has genotoxic action and damages DNA [8, 9]. In addition to its endocrine-disrupting effect, it has been shown that BPA causes cytokine disruption and oxidative stress in the liver and kidneys [10]. Moreover, patients with chronic renal illnesses have been found to have increased BPA plasma levels [11]. BPA-treated rats exhibit renal damage in the form of proteinuria, glomerular injuries, increased serum creatinine and decreased creatinine clearance, all of which are dose-dependent [12]. After BPA therapy, it is discovered that the urine tubules of the kidney have changed in structure[13].
Zinc (Zn) is a necessary nutrient for many physiological roles in humans and animals [14]. Zn is especially significant in cellular function since it is found in various proteins, including metalloenzymes, structural proteins, and transcription factors [15]. Zn supplementation reduces blood parameters such as hemoglobin concentration and hematocrit value [16]. Zn protects animals from the acute and sub-acute toxicity of metallic elements, including cadmium, chromium, and mercury [17]. Furthermore, Zn supplementation prevents the aggregation of reactive oxygen species (ROS), which is one of the leading causes of liver damage [18]. The transcription factor family Zn finger and homeobox (ZHX) highly occurs in podocytes and cause glomerular disease [19]. Zn supplementation significantly improves hepatic and renal function parameters, as well as histological changes linked with enzymatic and non-enzymatic antioxidant defense systems in arsenic-intoxicated animals [20].
Folic acid (FA) supplementation may lower stroke risk in areas where FA is not fortified, especially in trials with low FA and vitamin B12 levels [21]. This vitamin is critical for various bodily functions, including nervous system health, crucial nutrients for hematological components, and cell growth [22]. Blood folate levels increase the incidence of fatty liver in humans [23], perhaps through positive control of glucose metabolism and insulin resistance, or by catalyzing fat metabolism. FA supplementation decreased hepatic lipid accumulation and inflammatory foci aggregation caused by high-fat diet feeding [24]. FA therapy benefits CKD patients the greatest, with a 56 and 44% reduction in the risk of CKD development and the eGFR rate, respectively. In contrast, in those without CKD, the renoprotective effect was negligible [25]. FA supplementation may help to prevent or ameliorate the deleterious effects of methomyl exposure on renal histology and function [26].
Even though there are several reports on the hazardous effects of BPA and their amelioration by different approaches, there is a lack of studies on alleviating BPA toxicity by combined therapy of Zn and FA. The current study examined both individual and combinational treatments of Zn and FA to assess their potential protective effects against BPA-induced oxidative damages.
MATERIALS AND METHODS
Ethical approval
The Ethics Committee of Animal Welfare and Experimentation at Bangladesh Agricultural University authorized the trial. All mice were handled in accordance with the Animal Research Policy [Reference: AWEEC/BAU/2021(18)].
Animals and housing conditions
In the current study, 3.5-4 weeks aged 75 male mice weighing 25 ± 1.92 gm were utilized. The animals were housed in a compartmentalized square wooden cage wrapped with wire mesh under controlled conditions of temperature (26-30)°C and relative humidity of 70-80% with natural day light at the Department of Physiology, Bangladesh Agricultural University, Mymensingh 2202. All of the mice were provided with sufficient food and water.
Experimental approach
The experimental mice were randomly split into five groups (n = 15). Group A (control) was orally fed with normal mice pellets that are composed of all-natural powdered corn cob cellulose, while the treated groups were designed as follows: Group B (50 mg BPA/kg BW); Group C (50 mg BPA/kg BW+10 mg zinc sulfate/kg BW); Group D (50 mg BPA/kg BW+3 mg FA/kg BW); and Group E (50 mg BPA/kg BW+10 mg zinc sulfate/kg BW+3 mg FA/kg BW). All groups were fed orally-supplemented diets daily, and the trial continued for 12 weeks [27]. BPA was bought from SIGMA Company, Germany. Zinc and folic acid were collected from SQUARE company, Bangladesh.
Collection of blood
At the end of the trial, blood was taken by sacrificing the mice. Before blood collection, the mice were fasted the whole night and, followed by, kept one by one in an airtight desiccator containing diethyl ether. Meanwhile, they were inspected to see whether or not they were awake. After complete euthanization, about 1-1.5 ml of blood sample was directly collected from the heart using a sterile syringe from each mouse of a group.
Serum collection
Blood was taken directly from each mouse’s heart in Eppendorf tubes and allowed to clot. the serum was isolated from the clot, then centrifuged, and the collected clear serum samples which were stored at -20 °C to assess selected serum hepatorenal biomarkers.
Analysis of hematological parameters
The standard method suggested by Ghai [28] was used to measure the total erythrocyte count (TEC), hemoglobin concentration (Hb conc.), packed cell volume (PCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (Haematocrit), and mean corpuscular volume (MCV). These parameters were determined at the Department of Physiology, Bangladesh Agricultural University, Mymensingh.
Biochemical parameters
The collected serum was utilized for biochemical examination to analyze serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), creatinine, and total protein (TP) using a radioimmunoassay kit (Berthold, Germany).
Histotexture study
The liver and kidney samples were collected carefully and kept in 10% neutral buffered formalin for further histological analysis. The well-fixed tissue samples were processed to make paraffin block, precisely sectioned, and stained with hematoxylin and eosin (H&E) according to standard protocol [29] in collaboration with the Department of Pathology at Bangladesh Agricultural University. An Olympus photomicroscope (CX43) was used to analyze the stained slides.
Statistical analysis
All of the data were input and saved in Microsoft Excel 2019 (Microsoft, USA). Then, data were imported into GraphPad Prism v5.0 (GraphPad Software Inc., USA) for analysis utilizing a one-way analysis of variance (ANOVA) with a Bonferroni multiple comparison test. A P-value of ≤ 0.05 was considered statistically significant.
RESULTS
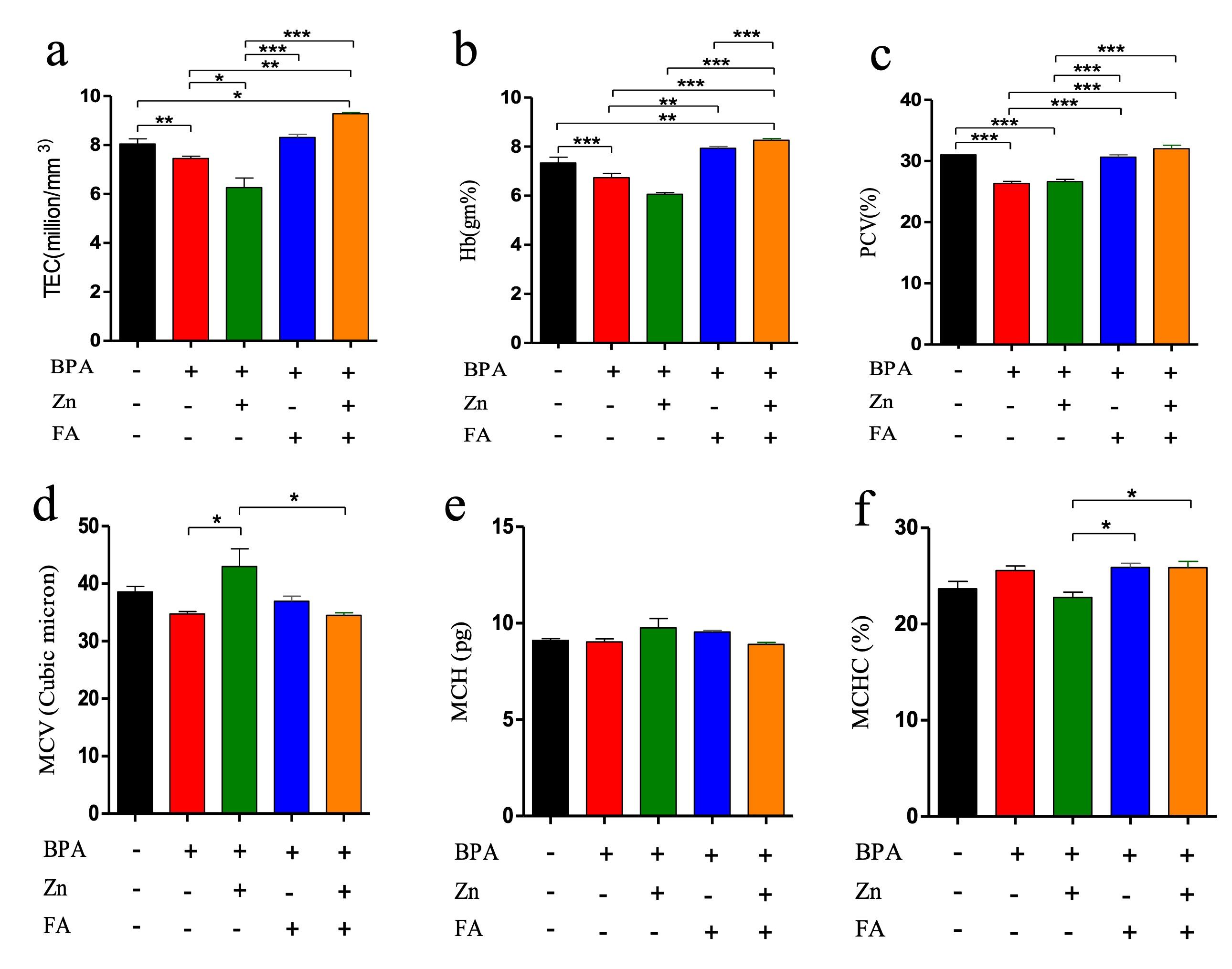
Effect of Zn and FA combination on hematological parameters
The blood parameters revealed that TEC, Hb conc., and PCV significantly (p≤ 0.05) decreased after BPA treatment (Figure 1). All these parameters were also reduced in the individually Zn-supplemented group, whereas individual FA supplementation elevated each of them. However, combinational supplementation of Zn and FA markedly increased (p≤ 0.05) TEC (Figure 1a), Hb conc. (Figure 1b) and PCV (Figure 1c) in comparison to the control mice. There were no significant alterations in MCV (Figure 1d), MCH (Figure 1e) and MCHC (Figure 1f) values in the treated groups compared to the control group.
Effects of Zn and FA combination on liver functions
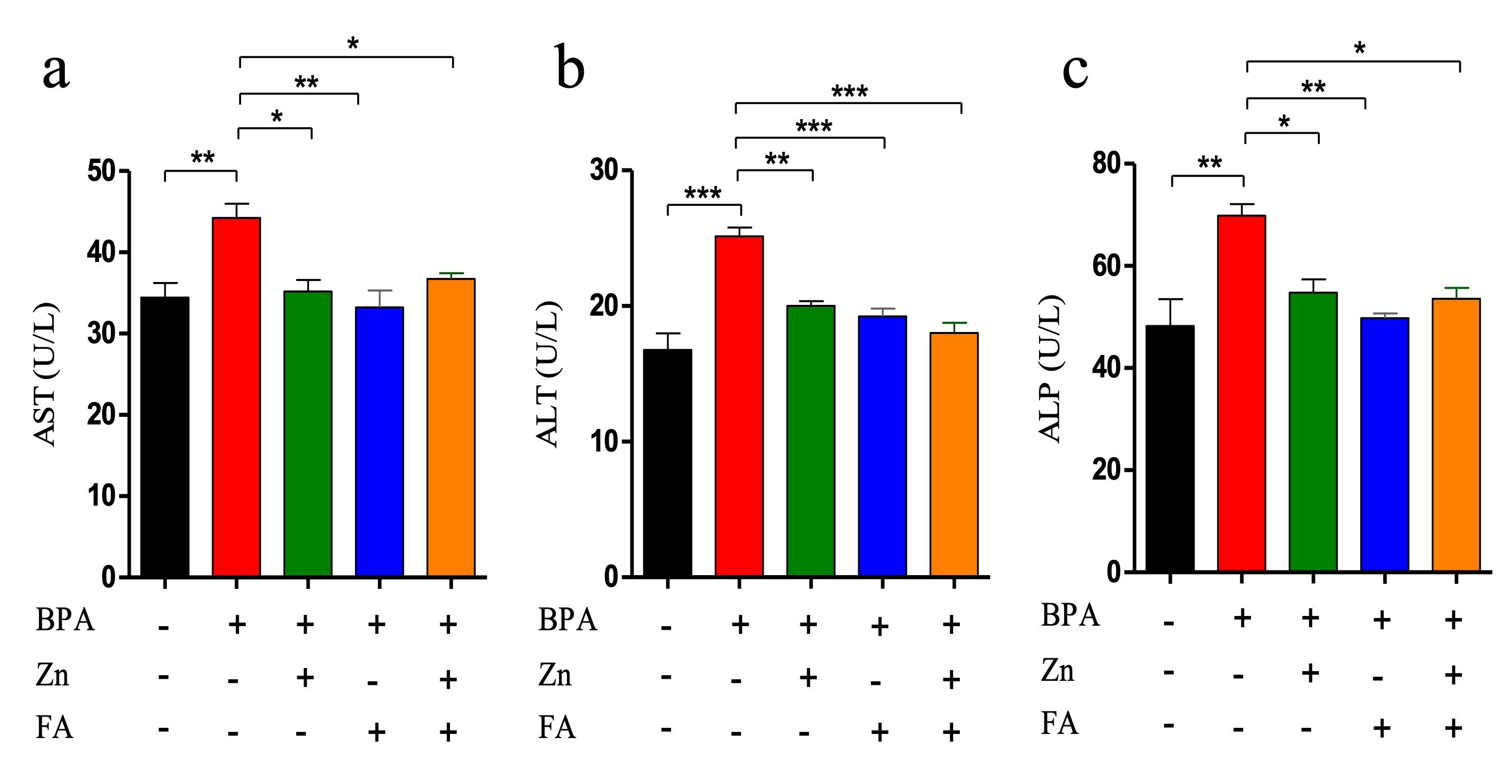
In the present study, BPA exposure significantly (p ≤ 0.05) elevated the level of three liver enzymes (ALT, ALP, and AST) compared to the control group (Figure 2). Individual supplementation of Zn and FA reduced (p ≤ 0.05) the AST (Figure 2a), ALT (Figure 2b) and ALP (Figure 2c) levels. However, compared to the control group, a significant (p ≤ 0.05) upregulation of all three liver enzyme levels was recorded in the BPA-treated group receiving combined supplementations of Zn and FA.
Effects of Zn and FA combination on liver injury
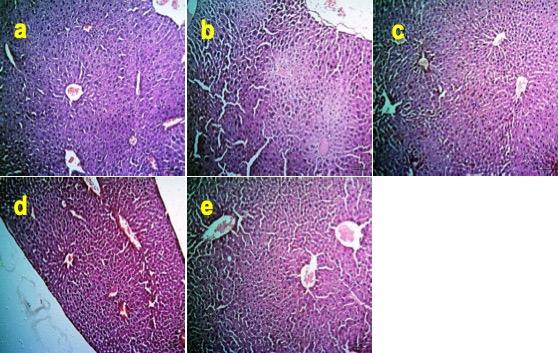
The liver of the BPA-treated mice showed severe degenerative and necrotic changes of hepatocytes (Figure 3b) compared to the control mice (Figure 3a). In contrast, individual supplementations of Zn, FA, or combinational supplementation of Zn and FA normalized the histoarchitecture of the liver (Figure 3c-e).
Effects of Zn and FA combination on kidney function
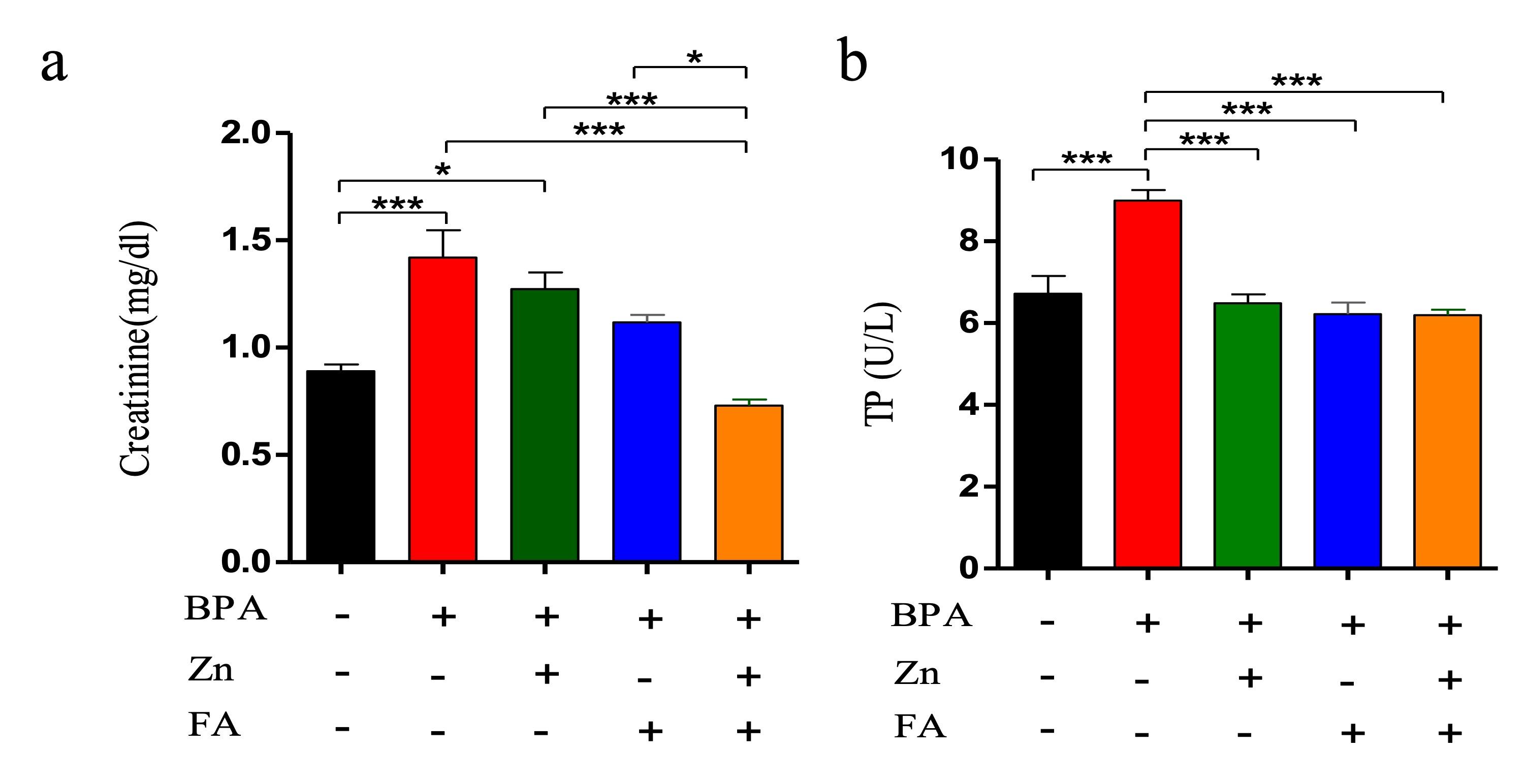
Serum creatinine (SCr) and total protein (TP) were analyzed in five groups of mice. An elevation of SCr and TP indicates kidney injury. In the current research, BPA exposure led to an increment in the level of SCr as well as TP compared to the control group (p≤ 0.05) (Figure 4). Individual supplementation of Zn and FA reduced (p≤ 0.05) the level of SCr (Figure 4a) and TP (Figure 4c) comparable to the control mice. However, a significant upregulation (p ≤ 0.05) of SCr and TP levels was observed in BPA-treated mice receiving combined supplementations of Zn and FA.
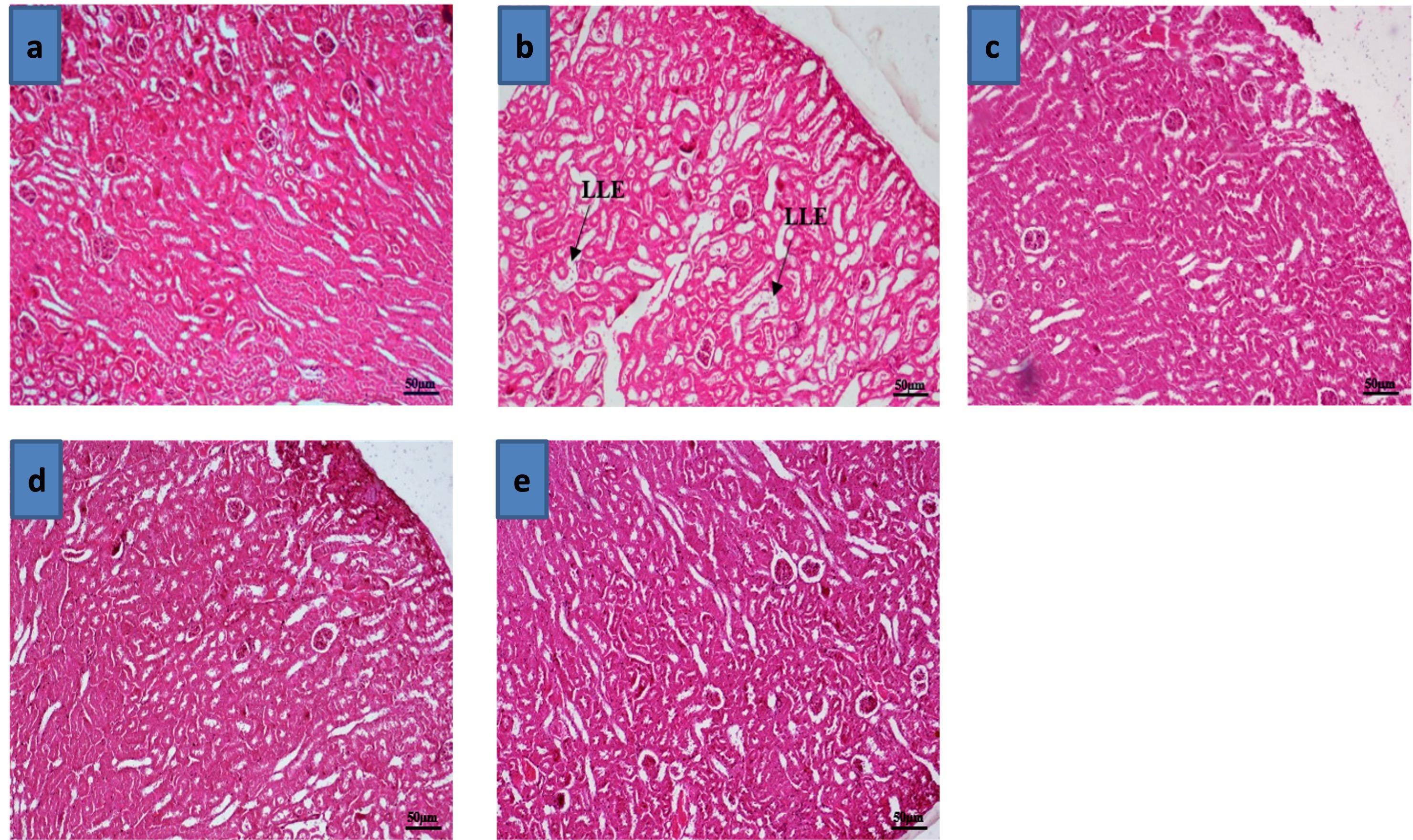
Effects of Zn and FA combination on kidney injury
Kidney of the BPA-treated mice revealed severe loss of lining epithelium of renal tubule (Figure 5b) compared to the control grouped mice (Figure 5a). However, individual supplementations of Zn, FA, or the combinational supplementation of Zn and FA restored the histoarchitectures of the kidney (Figure 5c-e).
DISCUSSION
In the cases of hematological parameters, the current study resulted that BPA exposure markedly lowered TEC, Hb concentration, and PCV, whereas Zn and FA supplementation increased TEC, Hb concentration, and PCV in BPA-exposed mice. However, no significant differences between the control and treatment groups were observed in MCV, MCH, or MCHC values. The results of BPA toxicity are aligned with several previous reports. Yamasaki and Okuda [30] discovered that BPA reduced RBC count, Hb concentration, and PCV in males but not MCV. BPA may reduce hemoglobin synthesis or RBC lysis, or it may change the structure of hemoglobin, affecting its physiological functions [31, 32]. In addition, BPA causes abnormal bone growth, decreased bone marrow mesenchymal stem cell survival, and osteogenic differentiation. These effects lead to insufficient RBC production [33]. However, the protective actions of Zn and FA might be explained by their vital role in the biosynthesis and regulations of hematological parameters. According to Kilic et al. [34], Zn, which is involved in many enzymatic processes, is also crucial for developing and synthesizing erythrocytes. Similarly, Folic acid is inevitable in developing blood cells [35]. Therefore, the necessity of Zn and FA for blood cell growth, development, and maturation, allows them to protect against hematobiochemical changes due to BPA exposure.
In this study, BPA exposure led to elevated activity of liver enzymes in mice with severe degenerative and necrotic hepatocyte changes, which were restored with Zn and FA supplements. A high ALT level was found in rats exposed to BPA for a reasonable period [36]. It is also reported that BPA exposure at both low and high concentrations can change liver enzyme profiles and cause hepatic damage [37]. In addition, BPA causes DNA damage in both human and rat hepatocytes [9]. According to Periasamy et al. [38], BPA exposure produces cellular infiltration, enlarges cytoplasmic vacuoles and hepatic sinusoids, and upregulates Kupffer cell number. Exposure to BPA also encourages oxidative damage [39]. In contrast, high doses of Zn are suggested to treat xenobiotic hepatotoxicity [40]. The antioxidant properties of Zn enable it to inhibit redox-active transition metals such as iron and copper and protect protein sulfhydryl groups from oxidative degradation [41]. Similarly, Folic acid has a sizable capacity to quench reactive oxygen radicals, protecting against oxidative damage [42].
Our research showed that exposing mice to BPA significantly increased serum creatinine and TP levels, indicating impaired renal function. Moreover, renal tubule lining epithelium loss in BPA-treated mice was significantly reduced, but it was reversible with Zn and FA supplements. BPA has a nephrotoxic impact because of the hazardous metabolite accumulation and the renal disability to expel them [43]. According to Asahi et al. [44], BPA encourages rough endoplasmic degradation and mitochondrial malfunction, which are crucial for the protein pathway. Serum Zn deficiency in diabetic patients exacerbates inflammatory processes, which, coupled with diabetes, produce and encourage kidney fibrosis. Zn possesses anti-inflammatory, anti-oxidative, and anti-fibrotic properties in diabetes [45]. Zn is vital in avoiding oxidative stress, apoptosis, and necrosis caused by toxicants, significantly reducing toxic damage to both liver and renal tissues with Zn supplementation [46]. Folic acid may protect against the detrimental effects of electromagnetic radiation exposure regarding the total glomeruli number [47]. But the actual synergistic effects of zinc and folic acid are unknown. Future studies will be needed to clarify the possible synergistic mechanisms of zinc and folic acid on BPA toxicities.
CONCLUSION
With the increasing exposure to BPA-like hazardous compounds, the strategies for mitigating their detrimental health effects are crucial. The investigation revealed that combination of Zn and FA supplementation significantly lowered the hazardous outcomes of BPA exposure on hematological parameters and the histostructure of the liver and kidney of male albino mice (Figure 6). Henceforth, the results suggest that Zn and FA supplementation could be a fruitful way to prevent or ameliorate toxicities caused by BPA. However, more thorough investigations are required to elucidate the protective mechanisms of Zn and FA supplementations against toxicity by BPA-like hazardous materials.
ACKNOWLEDGEMENT
The authors would like to express their gratitude to the Bangladesh Agricultural University Researcher System (BAURES) for funding the research (reference number 2019/44/BAU). The authors would also like to thank the Department of Pathology at Bangladesh Agricultural University in Mymensingh for providing laboratory assistance, as well as everyone engaged in the research for their eager engagement in this work.
AUTHORS CONTRIBUTION
AM conceived and carried out the research, analyzed the data, and drafted the manuscript; MA assisted in writing the article and analyzing the data. MAM revised the original draft and data analysis critically; KMS and AUM assisted in research planning and revised the initial draft; and EHC polished the manuscript, and oversaw the overall study activity.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Brotons JA, Olea-Serrano MF, et al. Xenoestrogens released from lacquer coatings in food cans. Environmental health perspectives. 1995;103:608-12.
- [2]Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2009;364:2063-78.
- [3]Tzatzarakis MN, Karzi V, et al. Bisphenol a in soft drinks and canned foods and data evaluation. Food additives & contaminants Part B, Surveillance. 2017;10:85-90.
- [4]Horiguchi H, Oguma E, et al. Suppression of erythropoietin induction by diethylstilbestrol in rats. Archives of toxicology. 2014;88:137-44.
- [5]Sharma P, Chadha P. Bisphenol a induced toxicity in blood cells of freshwater fish channa punctatus after acute exposure. Saudi journal of biological sciences. 2021;28:4738-50.
- [6]Korkmaz A, Ahbab MA, et al. Influence of vitamin c on bisphenol a, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2010;48:2865-71.
- [7]Kourouma A, Quan C, et al. Bisphenol a induces apoptosis in liver cells through induction of ros. Advan in Toxicol. 2015;2015.
- [8]Kabuto H, Amakawa M, et al. Exposure to bisphenol a during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life sciences. 2004;74:2931-40.
- [9]Kim S, Mun GI, et al. Submicromolar bisphenol a induces proliferation and DNA damage in human hepatocyte cell lines in vitro and in juvenile rats in vivo. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2018;111:125-32.
- [10]Morgan AM, El-Ballal SS, et al. Studies on the potential protective effect of cinnamon against bisphenol a- and octylphenol-induced oxidative stress in male albino rats. Toxicology reports. 2014;1:92-101.
- [11]González-Parra E, Herrero JA, et al. Bisphenol a in chronic kidney disease. International journal of nephrology. 2013;2013:437857.
- [12]Kobroob A, Peerapanyasut W, et al. Damaging effects of bisphenol a on the kidney and the protection by melatonin: Emerging evidences from in vivo and in vitro studies. Oxidative medicine and cellular longevity. 2018;2018:3082438.
- [13]Roig B, Cadiere A, et al. Environmental concentration of nonylphenol alters the development of urogenital and visceral organs in avian model. Environment international. 2014;62:78-85.
- [14]Mohamed ET, Said AI, et al. Protective effect of zinc aspartate against acetaminophen induced hepato-renal toxicity in albino rats. J Rad Res Appl Sci. 2011;4.
- [15]Zhou Z, Liu J, et al. Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Experimental biology and medicine (Maywood, NJ). 2008;233:540-8.
- [16]Kim J-H, Choi H, et al. Toxic effects on hematological parameters and oxidative stress in juvenile olive flounder, paralichthys olivaceus exposed to waterborne zinc. Aqua Repor 2019;15:100225.
- [17]Liu J, Kershaw W, et al. Rat primary hepatocyte cultures are a good model for examining metallothionein-induced tolerance to cadmium toxicity. In Vitro Cell Dev Biol 1990;26:75-9.
- [18]Zhou Z, Wang L, et al. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. The American journal of pathology. 2005;166:1681-90.
- [19]Macé C, Del Nogal Avila M, et al. The zinc fingers and homeoboxes 2 protein zhx2 and its interacting proteins regulate upstream pathways in podocyte diseases. Kidney international. 2020;97:753-64.
- [20]Garla R, Sharma N, et al. Effect of zinc on hepatic and renal tissues of chronically arsenic exposed rats: A biochemical and histopathological study. Biological trace element research. 2021;199:4237-50.
- [21]Zhao M, Wu G, et al. Meta-analysis of folic acid efficacy trials in stroke prevention: Insight into effect modifiers. Neurology. 2017;88:1830-8.
- [22]Koplay M, Gulcan E, et al. Association between serum vitamin b12 levels and the degree of steatosis in patients with nonalcoholic fatty liver disease. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2011;59:1137-40.
- [23]Polyzos SA, Kountouras J, et al. Serum vitamin b12 and folate levels in patients with non-alcoholic fatty liver disease. International journal of food sciences and nutrition. 2012;63:659-66.
- [24]Sid V, Shang Y, et al. Folic acid supplementation attenuates chronic hepatic inflammation in high-fat diet fed mice. Lipids. 2018;53:709-16.
- [25]Xu X, Qin X, et al. Efficacy of folic acid therapy on the progression of chronic kidney disease: The renal substudy of the china stroke primary prevention trial. JAMA internal medicine. 2016;176:1443-50.
- [26]Sakr S, Hassanien H, et al. Beneficial effects of folic acid on the kidneys and testes of adult albino rats after exposure to methomyl. Toxicology research. 2018;7:480-91.
- [27]Mustari A, Alam M, et al. Retrieval action of zinc and folic acid for the restoration of normal reproductive function in bisphenol-a exposed male albino mice. Vet med 2022;67:479-86.
- [28]Ghai CL. A textbook of practical physiology. In: 13th, editor. India: Jaypee brothers medical publisher (p) ltd2013. p. 211-8.
- [29]Luna LG. Manual of histologic staining methods of thearmed forces institute of pathology. New york: Mcgrow hill book co1968. p. 111-2.
- [30]Yamasaki K, Okuda H. Comparison of endocrine-mediated effects of two bisphenol a related compounds, 2,2-bis(4-cyanatophyenyl)propane and 4,4'-cyclohexylidenebisphenol, based on subacute oral toxicity studies using rats. Toxicology letters. 2012;208:162-7.
- [31]Fang X, Cao S, et al. Interaction of bisphenol a with bovine hemoglobin using spectroscopic and molecular modeling methods. Applied spectroscopy. 2011;65:1250-3.
- [32]Ulutaş OK, Yildiz N, et al. An in vivo assessment of the genotoxic potential of bisphenol a and 4-tert-octylphenol in rats. Archives of toxicology. 2011;85:995-1001.
- [33]Azimi AS, Lotfi B, et al. The effect of bisphenol a on osteogenic activity and morphology of rat bone marrow mesenchymal stem cells in vitro. In Vitro Cell. 2013;15.
- [34]Kilic M, Baltaci AK, et al. Effect of zinc supplementation on hematological parameters in athletes. Biological trace element research. 2004;100:31-8.
- [35]Mahmood LJJoHR, Reviews. The metabolic processes of folic acid and vitamin b12 deficiency. J of Health Res and Rev 2014;1:5.
- [36]Xia W, Jiang Y, et al. Early-life exposure to bisphenol a induces liver injury in rats involvement of mitochondria-mediated apoptosis. PloS one. 2014;9:e90443.
- [37]Moustafa GG, Ahmed AAM. Impact of prenatal and postnatal exposure to bisphenol a on female rats in a two generational study: Genotoxic and immunohistochemical implications. Toxicology reports. 2016;3:685-95.
- [38]Periasamy S, Chien SP, et al. Sesame oil mitigates nutritional steatohepatitis via attenuation of oxidative stress and inflammation: A tale of two-hit hypothesis. The Journal of nutritional biochemistry. 2014;25:232-40.
- [39]Maćczak A, Cyrkler M, et al. Bisphenol a, bisphenol s, bisphenol f and bisphenol af induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicology in vitro : an international journal published in association with BIBRA. 2017;41:143-9.
- [40]Stehbens WE. Oxidative stress, toxic hepatitis, and antioxidants with particular emphasis on zinc. Experimental and molecular pathology. 2003;75:265-76.
- [41]Powell SR. The antioxidant properties of zinc. The Journal of nutrition. 2000;130:1447s-54s.
- [42]Akgun E, Boyacioglu M, et al. The potential protective role of folic acid against acetaminophen-induced hepatotoxicity and nephrotoxicity in rats. Experimental animals. 2021;70:54-62.
- [43]Sangai NP, Verma RJ, et al. Testing the efficacy of quercetin in mitigating bisphenol a toxicity in liver and kidney of mice. Toxicology and industrial health. 2014;30:581-97.
- [44]Asahi J, Kamo H, et al. Bisphenol a induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life sciences. 2010;87:431-8.
- [45]Yang F, Li B, et al. The beneficial effects of zinc on diabetes-induced kidney damage in murine rodent model of type 1 diabetes mellitus. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS). 2017;42:1-10.
- [46]Mahran A, Osman H, et al. Protective effect of zinc (zn) on the histology and histochemistry of liver and kidney of albino rat treated with cadmium. J Cytol Histol. 2011;2:2-9.
- [47]Deniz Ö G, Kıvrak EG, et al. Effects of folic acid on rat kidney exposed to 900 mhz electromagnetic radiation. Journal of microscopy and ultrastructure. 2017;5:198-205.