Characterization of tetA gene of Escherichia coli isolated from colibacillosis affected calves in Rangpur, Bangladesh
Abstract
Antimicrobial resistance is a matter of threat in global public health nowadays as a great number of antibiotics have lost their potential. The current study aimed to characterize the tetA gene involved in tetracycline resistance of Escherichia coli in colibacillosis affected calves. To perform the study, a total of 69 fecal samples from colibacillosis suspected diarrheic calves were collected randomly from different areas of Rangpur division in Bangladesh. Standard laboratory protocols were followed for isolation and identification of E. coli. Then bacterial sensitivity was tested by culturing the bacteria in Mueller Hinton agar by following the protocol of Kirby-bauer disc diffusion method. Tetracycline resistant gene was specified by using tetA primer by searching tetA gene and the resistant gene was sequenced by sanger sequencing method. A phylogenetic tree of nucleotide sequences of tetA gene of E. coli was formulated using NCBI blast to characterize the gene. From 69 fecal samples, 54 were positive for E. coli. In antibiogram study, 100% isolates of E. coli were resistant to amoxicillin, ampicillin, cefoxitin, erythromycin and imipenem and the nature of the isolates of E.coli against other antibiotics were as follows: doxycycline 64.8%, co-trimethoprim 63%, tetracycline 59.3%, ceftriaxone 50%, gentamicin 16.7%, levofloxacin 14.8% respectively. This study noticed that about 56.25% samples (18 from 32) were resistant to tetracycline due to tetA gene and the gene (tetA from E. coli) was closely related to the corresponding gene of Klebsiella pneumoniae, Shigella sonnei, Proteus mirabilis and Citrobacter braakii strains besides E. coli. This study suggests determining the other genes that are specific for tetracycline resistance in E. coli in Rangpur for better understanding the nature of it.
INTRODUCTION
Calves are affected by a large number of infectious and non-infectious diseases. Among these, Escherichia coli causing colibacillosis is regarded as one of the most important problems in the dairy industry which has a negative impact on a nation’s economy [1]. From environmental sources, E. coli colonizes in the mammalian intestinal tract just after birth and these organisms remain as important members of the normal intestinal flora throughout life [2]. E. coli affects calves below 2 months old and the bacteria are prevalent in farms according to the farm location, calf management, and herd size [3, 4]. Colibacillosis-affected calves normally show diarrhea, dehydration, metabolic acidosis and fever with meningitis and arthritis can occur as a sequel in some cases. However, typical signs noticed in colibacillosis are fever, diarrhea, and dehydration [5].
Although E. coli is normally present in the intestine as an essential flora, a variety of enteric infections (colibacillosis is one of them) can occur which can lead to life-threatening conditions hence antimicrobial therapy is needed [6]. But most of the time, the farm owners use antibiotics without consulting any registered veterinarians, and the animals are not tested whether they have relevant disease or not. Moreover, the overuse of antibiotics in animals is a very important cause of antimicrobial resistance. The indiscriminate use of antimicrobial agents by farm owners is a common malpractice triggering the progression of multi-drug resistant bacteria [7].
Several studies suggest that penicillin, tetracyclines, sulfa drugs, and cephalosporins are at risk for antimicrobial resistance [8, 9]. Antibiotic resistance is interlinked between humans and farm animals because resistant organisms can easily be transmitted to humans in different ways. Therefore, antimicrobial resistance in animals is one of the biggest issues of the 21st century [10]. Every year, near about 0.7 million humans are gone towards death throughout the world of it. This number can be enhanced in the future if effective antibiotics are not discovered [11]. So, it creates attention for healthcare personnel as well as economic experts to prevent the acquired antibiotic resistance [12].
Specific genes of micro-organisms are responsible for each of the antibiotic resistance. E. coli has numerous genes that are responsible for resistance to beta-lactam antibiotics. Narrow-spectrum β-lactamases coded by blaTEM-1 can inactivate penicillin and aminopenicillins and this gene is widespread in E. coli from animals [13]. Tetracycline resistance is predominant in E. coli due to tet genes including tetA, tetB, tetC, tetD, tetE, and tetG where 80% of E. coli isolates are resistant due to tetB gene [14]. Although the amplitude of tetracycline-resistant genes between tetA and tetB is fluctuating from one form to another, tetA gene is predominant than tetB gene in Bangladesh [15, 16]. Previously, very limited study focused on determining resistant genes responsible for tetracycline resistance in Bangladesh. This study aimed to characterize tetracycline resistant gene of E. coli due to tetA gene. The rest of the genes which are engaged in tetracycline resistance should be characterized in future to fulfill the intention of the present study.
MATERIALS AND METHODS
Ethical approval
The ethical approval was provided by Hajee Mohammad Danesh Science and Technology University (HSTU/IRT/4215).
Collection and processing of samples
From January 2022 to December 2022, 40 farms in the selected areas were continuously supervised to collect 69 fecal samples from colibacillosis suspected calves with fever, diarrhea, and general weakness in different parts of Rangpur, Bangladesh. Among those samples, 41 were collected from males, and 28 were collected from females. In terms of age, the sample size of <1 week, 1-3 weeks and 3-6 weeks were 21, 33, and 15 respectively. The samples were taken immediately after defecation or directly from the rectum by using sterile gloves. Then the samples were taken in a dry and clean special vial containing phosphate buffered solution (HI MEDIA, Mumbai, India) with tagging relevant information. The samples were fresh and transferred immediately from the farms to the laboratory in an ice box containing ice gel [17, 18]. In the laboratory, fecal samples were stored at 4°C until the preparation of culture media. All the samples were inoculated within the clean bench by maintaining an aseptic procedure. Sample collection to inoculation was done within 24 h [19].
Bacterial culture and biochemical tests
By using a sterile platinum loop, the collected fecal samples were directly inoculated by streaking onto nutrient agar media (HI MEDIA, Mumbai, India) and incubated at 37°C for 24 h. Later some colonies from nutrient agar media were streaked onto MacConkey agar (HI MEDIA, Mumbai, India) and then Eosin Methylene Blue (EMB) agar media (HI MEDIA, Mumbai, India) while incubating at 37°C for 24 h. The colonies from EMB agar were sub-cultured in the same media several times to produce a pure culture of E. coli [20]. After that, the bacterium was identified by performing gram’s staining technique and several biochemical tests like as catalase, oxidase, methyl red, Voges Proskauer (VP), indole, citrate utilization, triple sugar iron (TSI), motility-indole-urease (MIU) according to the former study [21]. Here all the reagents used in the biochemical test were collected from HI MEDIA, Mumbai, India.
Amplification of 16s and 23s rRNA gene of E. coli
For molecular confirmation, the 16s and 23s rRNA genes of E. coli were targeted by Eco-223 and Eco-455 primers designed through polymerase chain reaction (PCR) in a thermocycler (Biotech, India) as shown in Tables 1 and 2. To do this, a heating method was followed for extracting desired DNA from E. coli [22]. Previous protocols were followed for setting PCR conditions and gel documentation where GoTaq® Green master mix, 2x (Promega, USA) was used [23, 24].
Antibiotic-resistant profiling
Antibiotic sensitivity test was performed according to Kirby-Bauer disc diffusion method [25]. Pure culture from EMB agar plates was cultured onto Mueller-Hinton agar (HI MEDIA, Mumbai, India) by streaking with different antibiotic discs (amoxicillin, ampicillin, ceftriaxone, cefoxitin, streptomycin, gentamicin, erythromycin, tetracycline, doxycycline, chloramphenicol, ciprofloxacin, enrofloxacin, levofloxacin, imipenem, and co-trimethoprim) and incubated at 37°C for 24 h. All the antibiotic discs were imported from HI MEDIA, Mumbai, India. The zone of inhibition was calculated to millimeter unit and compared with clinical and laboratory standard institute (CLSI), 2023 to make antibiotic-resistant profile of E. coli [26].
PCR amplification of tetA gene
DNA extracts from 32 tetracycline-resistant isolates of E. coli were taken for tetA gene identification. tetA gene responsible for tetracycline resistance was identified by using tetA primer through PCR amplification. PCR reaction for detection of tetA gene was performed by setting the standard PCR condition with the following steps: initial denaturation for a single cycle with repeated 30 cycles each having denaturation, annealing, and final extension for a single cycle (Table 1 and 2). That was performed according to the method described formerly by several authors [27, 28]. The amplified PCR product was run through 1.5% agarose gel containing ethidium bromide at 85 volts for 45 minutes. After the dye line ran approximately 75-80% of the gel, DNA fragments were visualized by using a gel documentation machine (UVITEC, United Kingdom) which emits UV light.
Table 1. Primers used in the present study.
Table 2. Conditions of PCR for Eco-223, Eco-455 and tetA primers.
Sequencing tetA gene and analysis of the phylogenetic tree
According to the previous study, the tetA gene was sequenced (2 PCR products were used) by Sanger sequencing method by using tetA forward primer [29]. The sequenced data were edited, and gene fragments were assembled in the BioEdit software, and the data were submitted to the gene bank. The accession number provided by the gene bank was OR450016. The tetA sequences were performed blast on the NCBI website. Additional tetA gene sequences were retrieved from the Genbank and included in the data set. Multiple sequence alignments were done using ClustalW performed in the BioEdit software package. MEGA11 software was used for the construction of a phylogenetic tree and multiple sequence alignment was performed to align the sequences using the MUSCLE algorithm. The Neighbor-Joining root method was used with the maximum composite likelihood nucleotide substitution mode. 500 Bootstrap validity score was considered for the construction of the tree.
RESULTS
Properties of E. coli in cultural and biochemical studies
After incubation at 37°C for 24 h, a rose-pink colony appeared in MacConkey agar media, and in EMB agar plates, the colony showed a characteristic green metallic sheen (Figure 1). After several sub-cultures, the organism was found as a small rod under a microscope using the Gram staining technique. In biochemical tests, the isolates were positive for catalase, methyl red, indole, motility test, and gas production and negative for VP, citrate utilization, and urease test (Figure 2). 232 bp band was noticed in UV exposure after PCR amplification that ensured the presence of E. coli (Figure 3). Out of 69 fecal samples, 54 were positive for E. coli based on cultural, biochemical, and PCR results.
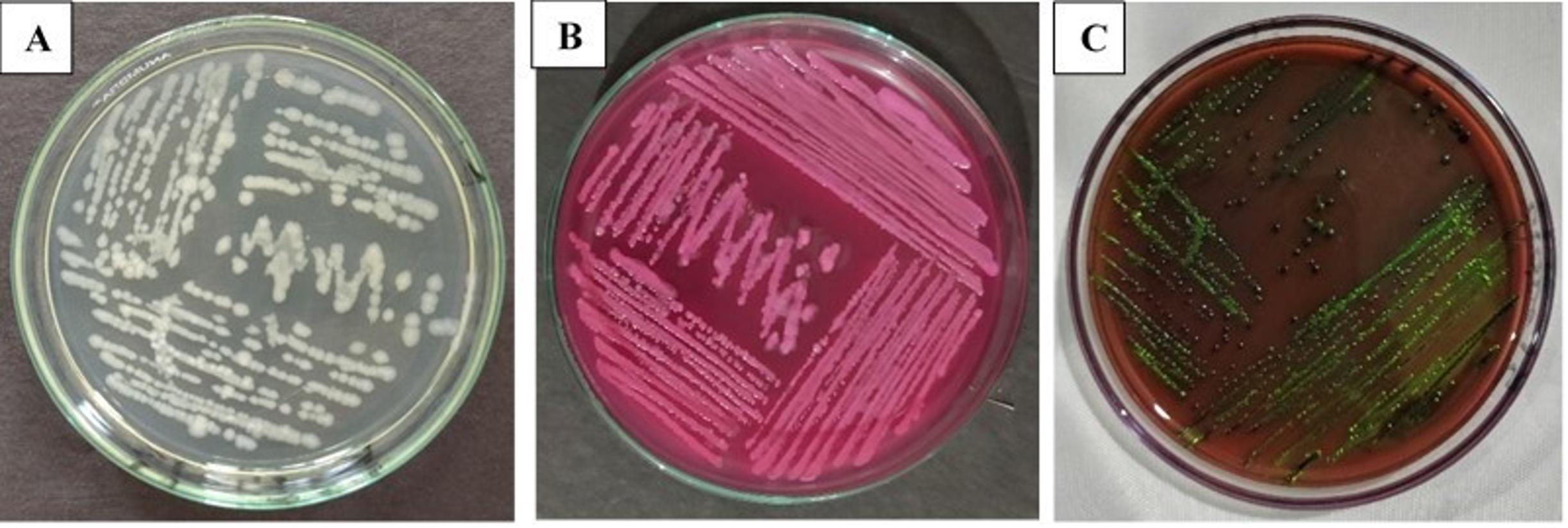
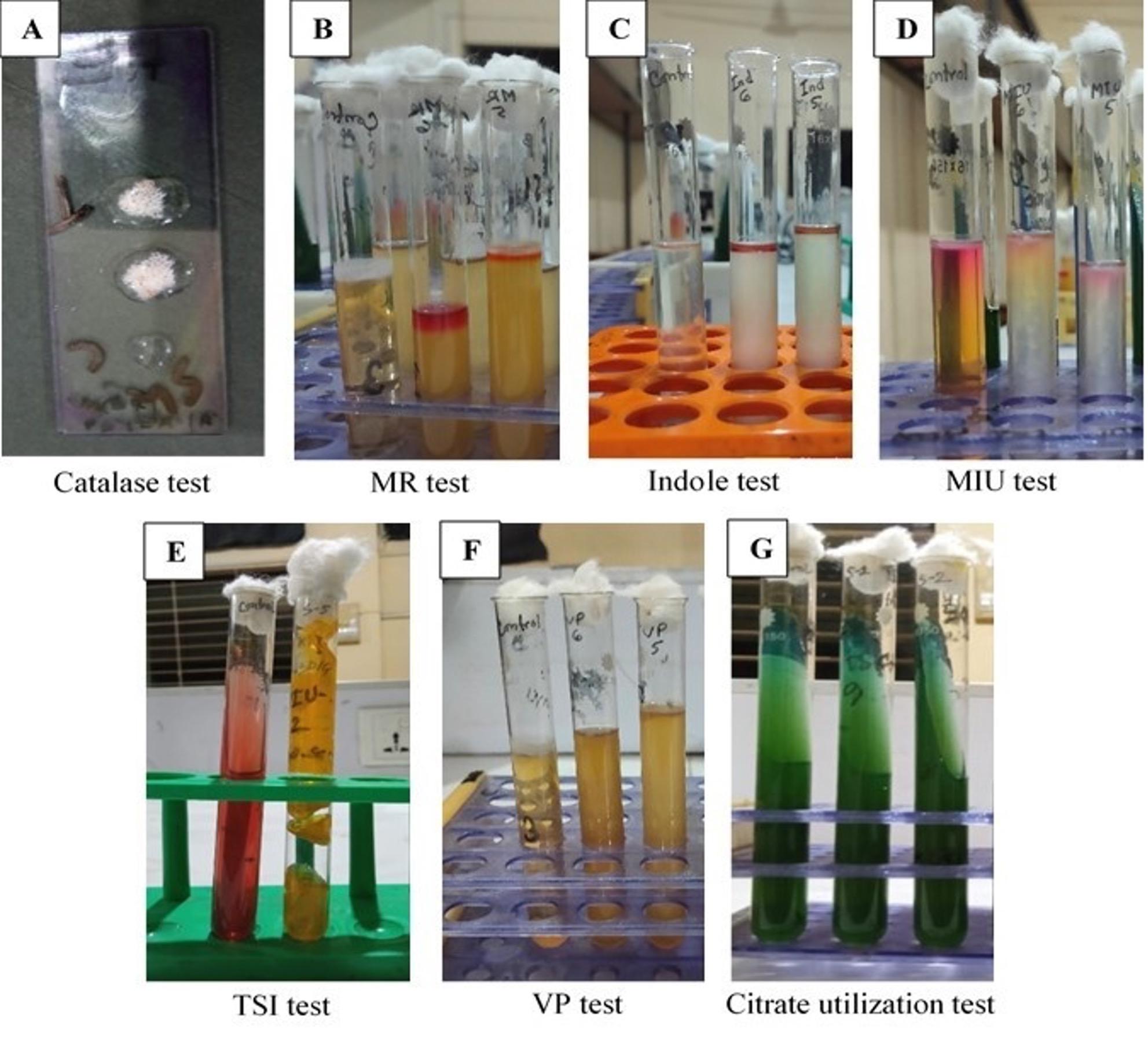
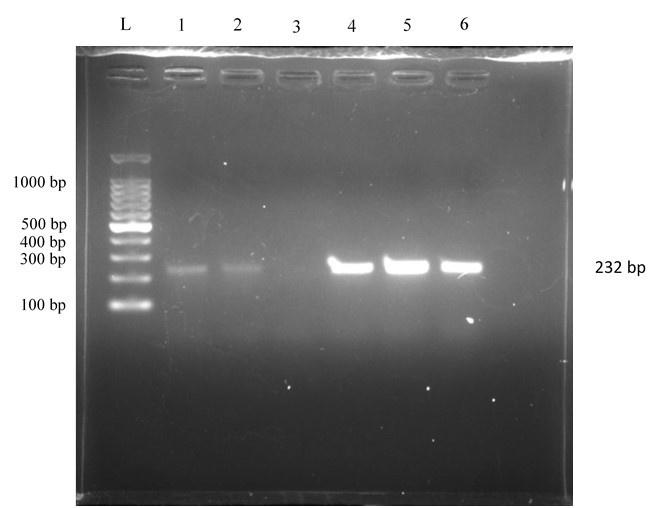
Antibiotic resistance profile of E. coli
54 samples were tested by the disc diffusion method for antibiotic sensitivity. 100% of the isolates of E. coli were resistant to amoxicillin, ampicillin, cefoxitin, erythromycin, and imipenem, and the resistant pattern against other antibiotics were as follows: doxycycline (64.8%), co-trimethoprim (63%), tetracycline (59.3%), ceftriaxone (50%), gentamicin (16.7%), chloramphenicol (16.7%), and levofloxacin (14.8%). The overall results of antibiotic sensitivity are shown in Table 3.
Table 3. Antibiotic resistant profile of E. coli.
Prevalence of tetA gene
PCR amplification was done for the purpose of identifying the tetA gene responsible for tetracycline resistance. 32 samples (the isolates that were visually resistant against tetracycline in the disc diffusion method) were run under PCR, in which 18 samples were positive (577 bp band was visualized) for the tetA gene, indicating that 56.25% of isolates of E. coli were resistant against tetracycline due to the tetA gene (Figure 4). Of 18 positive isolates, 5 were collected from the <1-week group, 9 were collected from the 1-3-week group, and 4 were collected from the 3-6-week group.
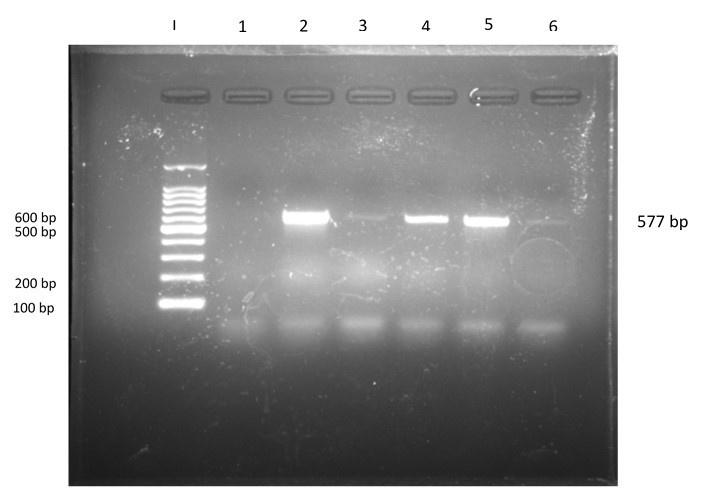
Clade of tetA gene in phylogenetic tree analysis
To further investigate the genetic relationship of the E. coli tetA gene, a phylogenetic tree was constructed. According to Figure 5, the gene sequence was homologous to E. coli strain GN03204 plasmid p3204 as well as the strains formerly observed at HSTU, including E. coli strain G1097 plasmid, E. coli strain XYEH2934 plasmid, and E. coli strain MS1050 plasmid. Besides E. coli, the gene sequence was also closed to the genes of Shigella sonnei strain 202008564 plasmid, Proteas mirabilis strain SDM004 plasmid pSDM004, Klebsiella pneumoniae strain TSNTC10-1 plasmid, and Citrobacter braakii strain LBA3.

DISCUSSION
Calf diarrhea, mainly calf colibacillosis, in Rangpur is a major problem in the dairy industry like the other division of Bangladesh. In most cases, the farm owner tends to use antibiotics without maintaining proper dosage [30]. As a result, antibiotic resistance is a common issue in Rangpur nowadays. Antibiotics are mainly used to treat bacterial infections in humans and animals as they have the ability to kill the bacteria or suppress their growth. Penicillin was discovered almost 100 years ago but in the present time, physicians feel they lack the weapons to fight infections [31]. This study aimed to study the antibiogram pattern of E. coli causing colibacillosis by determining the role of the tetA gene in the resistance of E. coli against tetracycline.
To observe the matter, diarrheal samples from colibacillosis-affected calves were tested for E. coli existence. The samples were cultured on different media (Nutrient agar, MacConkey agar, and EMB agar) to obtain a pure culture of E. coli. A rose pink and green metallic sheen colored colony was found in MacConkey and EMB agar, which was supported by the former studies [32, 33]. Some colonies were taken from EMB agar with a sterile platinum loop and made a thin smear on glass slide to perform gram’s staining technique where the organism was visible as pink colored rod indicating gram negative organism most probably E. coli and that was identical to previous study [34]. From EMB and nutrient broth, different biochemical tests were performed to confirm the accuracy of E. coli. The bacterial cultures were positive to catalase, methyl red, indole, motility test & gas production and were negative to VP, citrate utilization, urease as well as oxidase test supported by the former researcher [35].
After E. coli confirmation, an antibiotic-resistant profile was made. In this study, 100% of the isolates of E. coli are resistant to amoxicillin, ampicillin, cefoxitin, erythromycin, and imipenem, which indicates a mammoth threat to the health sector in the near future. This study also tried to examine E. coli against some other important antibiotics, and the results are as follows: doxycycline (64.80%), co-trimethoprim (63.00%), tetracycline (59.30%), ceftriaxone (50.00%), gentamicin (16.70%), and levofloxacin (14.8%). Previously, several studies had been done where they found the same in the cases of amoxicillin and ampicillin, but they found E. coli 43.18% resistant to erythromycin. On the contrary, the isolates of E. coli were 100% resistant to ampicillin, tetracycline, co-trimoxazole, and erythromycin [36, 37].
Previously, many scientists tried to find out the prevalence of different genes associated with tetracycline resistance in E. coli. In 2005–06, researchers found 54.4% of isolates of E. coli were resistant to tetracycline, 8.1% of which occurred due to the tetA gene and 86.5% due to the tetB gene. In 2010–11, E. coli resistance was 47.1% to tetracycline, and at that time, a clear fluctuation between the tetA and tetB genes was noticed. tetA was more dominant than tetB, and the percentage was 81.3% for tetA and 18.7% for the tetB gene [16]. The present study notices that 59.3% of isolates of E. coli are resistant against tetracycline, and this is higher than the previous record. The prevalence of the tetA gene is 56.25%, which is intermediate between the results of the two studies stated before. The same study was performed in 2019, where tetracycline was 100% resistant to E. coli and 77.80% of isolates were positive for the tetA gene [38]. In phylogenetic analysis, the tetA gene of E. coli isolated from colibacillosis-affected calves in Rangpur, Bangladesh, found the same clade as other E. coli plasmids from the animals.
As a multiple number of genes are responsible for the resistance of E. coli to tetracycline, demarking a single gene (tetA) cannot express the full idea of its nature. So, it should be emphasized to detect the other genes to accomplish the study goal.
CONCLUSION
From the present findings, the isolates of E. coli were resistant to multiple antibiotics; E. coli was found to be 100% resistant to amoxicillin, ampicillin, cefoxitin, erythromycin, and imipenem, indicating an alarming threat in the near future. 59.3% of E. coli isolates were found resistant to tetracycline in the disc diffusion method, while 56.25% of isolates (18 out of 32) were resistant due to the tetA gene. After confirmation of the tetA gene by PCR amplification, the tetA gene responsible for tetracycline resistance was sequenced, and this concludes that the sequenced gene was nearly similar to the gene present in the strains Klebsiella pneumoniae, Shigella sonnei, Proteus mirabilis, and Citrobacter braakii, besides E. coli, hence warning that a similar mechanism was present among them for tetracycline resistance. This study suggests antibiotics should be used only after sensitivity testing with proper dosage in order to avoid antibiotic resistance.
ACKNOWLEDGEMENT
The author would like to acknowledge the Ministry of Science and Technology, Government of the People's Republic of Bangladesh, for their tangible support as they selected the author for an NST scholarship to conduct the research work fruitfully (NST 2021-22/R225/M314). The author is also thankful to his research supervisor, Md Mustafizer Rahman, and co-supervisor, Nazmi Ara Rumi, for their expertise, active supervision, inspiration, and valuable suggestions to complete the research work.
AUTHOR CONTRIBUTIONS
MMR and NAR provided the study design and concept; JHJ and NAR performed the laboratory work (both gross and molecular); JJH and NAR wrote down the main manuscript; and MMR reviewed the manuscript. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Bashahun GM, Amina A. Colibacillosis in calves: A review of literature. Journal of Animal Science and Veterinary Medicine. 2017; 2: 62-71.
- [2]Quinn PJ, Markey BK, et al. Pathogenic bacteria-Enterobacteriaceae. Concise Review of Veterinary Microbiology. John Wiley & Sons: West Sussex, UK, 2016, pp 59.
- [3]Guler L, Gunduz K. Virulence properties of Escherichia coli isolated from clinical bovine mastitis. Turk J Vet Anim Sci. 2007; 31 :361-365.
- [4]Picco NY, Alustiza FE, et al. Molecular screening of pathogenic Escherichia coli strains isolated from dairy neonatal calves in Cordoba province, Argentina. Revista Argentina de Microbiologia. 2015; 47: 95-102.
- [5]Radostits OM, Gay CC, et al. Veterinary medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. Elsevier Health Sciences: Edinburg, Texas, USA, 2007.
- [6]Tyson GH, McDermott PF, et al. WGS accurately predicts antimicrobial resistance in Escherichia coli. Journal of Antimicrobial Chemotherapy. 2015; 70: 2763-2769.
- [7]Tawyabur M, Islam MS, et al. Isolation and characterization of multidrug-resistant Escherichia coli and Salmonella spp. from healthy and diseased turkeys. Antibiotics. 2020; 9: 770.
- [8]Galland JC, Hyatt DR, et al. Prevalence, antibiotic susceptibility, and diversity of Escherichia coli O157:H7 isolates from a longitudinal study of beef cattle feedlots. Appl Environ Microbiol. 2001; 67: 1619–27.
- [9]Van den Bogaard AE, London N, et al. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. Journal of Antimicrobial Chemotherapy. 2001; 47: 763-771.
- [10]Tacconelli E, Pezzani MD. Public health burden of antimicrobial resistance in Europe. The Lancet Infectious Diseases. 2019; 19: 4-6.
- [11]Clifford K, Desai D, et al. Antimicrobial resistance in livestock and poor-quality veterinary medicines. Bulletin of the World Health Organization. 2018; 96: 662-664.
- [12]Tirumalai MR, Karouia F, et al. Evaluation of acquired antibiotic resistance in Escherichia coli exposed to long-term low-shear modeled microgravity and background antibiotic exposure. Mbio. 2019; 10: e02637-18.
- [13]Poirel L, Madec JY, et al. Antimicrobial resistance in Escherichia coli. Microbiology Spectrum. 2018; 6: 6-4.
- [14]Wilkerson C, Samadpour M, et al. Antibiotic resistance and distribution of tetracycline resistance genes in Escherichia coli O157: H7 isolates from humans and bovines. Antimicrobial Agents and Chemotherapy. 2004; 48: 1066-1067.
- [15]Rafiq K, Islam MR, et al. Antimicrobial Resistance Profile of Common Foodborne Pathogens Recovered from Livestock and Poultry in Bangladesh. Antibiotics. 2022; 11: 1551.
- [16]Skockova A, Cupakova S, et al. Detection of tetracycline resistance genes in Escherichia coli from raw cow’s milk. Journal of Microbiology, Biotechnology and Food Sciences. 2021; 2021: 777-784.
- [17]Su W, Du Y, et al. Standards for collection, preservation, and transportation of fecal samples in TCM clinical trials. Frontiers in Cellular and Infection Microbiology. 2022; 12: 783682.
- [18]Santiago A, Panda S, et al. Processing faecal samples: a step forward for standards in microbial community analysis. BMC Microbiology. 2014; 14: 1-9.
- [19]Jorgensen JH, Pfaller MA. Introduction to the 11th edition of the manual of clinical microbiology. Manual of Clinical Microbiology. Wiley: Hoboken, New Jersey, USA, 2015, pp 1-4.
- [20]Brown A, Smith H. Benson’s Microbiological Applications: Laboratory Manual in General Microbiology. McGraw-Hill Education: 2 Penn Plaza, New York, USA, 2014, pp 247-249.
- [21]Cheesbrough M. Biochemical tests to identify bacteria. Medical Laboratory Manual for Tropical Countries. Cheesbrough: Doddington, Cambridgeshire, 2006, pp 62-70.
- [22]Dashti AA, Jadaon MM, et al. Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009; 41: 117-22.
- [23]Riffon R, Sayasith K, et al. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. Journal of Clinical Microbiology. 2001; 39: 2584-2589.
- [24]Le PY, Costumbrado J, et al. Agarose gel electrophoresis for the separation of DNA fragments. JoVE (Journal of Visualized Experiments). 2012; 62: e3923.
- [25]Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. American Society for Microbiology. 2009; 15: 55-63.
- [26]Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute (CLSI). 33rd ed. 2023; 58-71.
- [27]Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. Journal of Infection. 2009; 59: 4-16.
- [28]Abdelgader SA, Shi D, et al. Antibiotics resistance genes screening and comparative genomics analysis of commensal Escherichia coli isolated from poultry farms between China and Sudan. BioMed Research International. 2018; 2018.
- [29]McCombie WR, McPherson JD, et al. Next-generation sequencing technologies. Cold Spring Harbor Perspectives in Medicine. 2019; 9: 036798.
- [30]Sharma G, Mutua F, et al. A qualitative study on antibiotic use and animal health management in smallholder dairy farms of four regions of India. Infection Ecology & Epidemiology. 2020; 10: 1792033.
- [31]Serwecińska L. Antimicrobials and antibiotic-resistant bacteria: a risk to the environment and to public health. Water. 2020; 12: 3313.
- [32]Thapa DB, Chapagain A. Antibiogram of Escherichia coli isolated from avian colibacillosis in Chitwan district of Nepal. International Journal of Applied Sciences and Biotechnology. 2020; 8: 52-60.
- [33]Ema FA, Shanta RN, et al. Isolation, identification, and antibiogram studies of Escherichia coli from ready-to-eat foods in Mymensingh, Bangladesh. Veterinary World. 2022; 15: 1497.
- [34]Sultana T, Rabbi MF, et al. Prevalence and antibiotic resistance patters of Escherichia coli isolated from milk and milk product. Journal of Bio-Science. 2021; 29: 81-91.
- [35]Thaker HC, Brahmbhatt MN, et al. Study on occurrence and antibiogram pattern of Escherichia coli from raw milk samples in Anand, Gujarat, India. Veterinary World. 2012; 5: 556.
- [36]Abdullah M, Akter MR, et al. Characterization of bacterial pathogens isolated from calf diarrhoea in Panchagarh district of Bangladesh. J. Agric. Food. Tech. 2013; 3: 8-13.
- [37]Mailk S, Kumar A, et al. Incidence and drug resistance pattern of collibacillosis in cattle and buffalo calves in Western Uttar Pradesh in India. J. Anim. Health Prod. 2013; 1: 15-19.
- [38]Nahar A, Islam MA, et al. Detection of tetracycline resistant E. coli and Salmonella spp. in sewage, river, pond and swimming pool in Mymensingh, Bangladesh. African Journal of Microbiology Research. 2019; 13: 382-7.