COVID-19: The catastrophe of our time
Abstract
The most discussed topic in today’s world is COVID-19, an acute respiratory disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) because of its contagious transmission pattern, and morbimortality. The virus was originated by bats and in December 2019, first spread to humans by unknown intermediate species in Wuhan, China. The dramatic acceleration of the occurrence and death toll of COVID-19 with no potential medicine and vaccine are enough to explain its severity. This review summarizes multidisciplinary aspects of COVID-19, including origin, epidemiology, symptoms, transmission, pathogenicity, impact on world economy and advances in the use of modern diagnostic procedures and methods. Further, we analyzed extensively for various therapeutic strategies, potential drug options with prospective vaccine candidates and challenges along the way. All data were accumulated through extensive study of recent peer-reviewed publications and authentic reports until June 7, 2020. Collectively, this review would help to shed light on different dimensions of this ongoing pandemic.
INTRODUCTION
The whole world is silenced, and the streets get cleansed in a matter of brief period, and the name responsible for such act is 2019 novel coronavirus (2019-nCoV) or the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). After the emergence of COVID-19 on December 31, 2019, in Wuhan, this outbreak hit all 30 EU/EEA countries and the United Kingdom (UK) within March 15, 2020 [1]. In course of time, April 6, 2020, Spain, Italy, the United States, France and the United Kingdom have been infected catastrophically [2]. According to worldometer (https://www.worldometers.info/coronavirus/), this pandemic spread over 215 countries and territories by June 7, 2020, and confirmed death cases were 402,564 where over 70,000 people died by last two weeks which indicate its ferocity [3]. Developed countries like the United States are now in the shortage of personal protective equipments (PPE) including gloves, face shields, gowns, and hand sanitizer for frontline healthcare workers and high rates of infection are observed among healthcare associates in Italy [4]. As there is no preventive method, lack of intensive care unit (ICU), ventilator, and diagnosing availability could simply raise the mortality rate radically [5].
The origin of the pandemic is still hypothesized, though the genomic identity of coronavirus isolated from human, and the horseshoe bat Rhinolophus affinis showed enormous similarities [6]. It is reported that human is the only reservoir of the virus. Aside from droplet and contact transmission, some cases suggest aerosol and fecal-oral transmission which needs further verification [7, 8]. After the transmission of SARS-CoV-2, it generally takes 2-14 days for the viral incubation, and symptoms are expressed afterward [9]. As the incubation period, is asymptomatic, the affected person can transmit virus without knowing, which is one of the major reasons for COVID-19 being a pandemic. This evidence of transmission by the asymptomatic carrier has already been found in a case study, described a 20-year-old woman from Wuhan, China, who passed the coronavirus to five family members but never got physically sick herself [10].
In recent times, the world has reintroduced with some concepts like social/physical distancing, quarantine, lockdown and isolation. As there is yet no validated preventive method, social/physical distancing is the best possible way to limit the infection rate. Through locking down people and contact tracing the infected ones, necessary steps can be taken and that’s why rapid diagnosis in mass scale is a necessity [11]. Though several molecular and serological assays are developed as diagnostics, shortage of reagents, lack of availability of diagnosis, the rapidness of result generation and accuracy of the generated result are creating chaos and anarchy [12].
Until now, there is no Food and Drug Administration (FDA) licensed drugs for the treatment of COVID-19, but in recent times several drugs, for example, Chloroquine, Remdesivir, Lopinavir–Ritonavir have received attention [13]. Despite the multifaceted challenges, several pharmaceuticals, institutions and universities have embarked on the development of vaccines against SARS-CoV-2, the best possible way to combat this pandemic [14].
Given the current circumstances, the scientific community is acting rapidly to bring an end to this pandemic. This review aims to provide evidence of early findings, epidemiology, transmission, pathogenesis, risk factors, diagnosis, economic impact as well as suggesting potential drug options, challenges and prospects to develop the COVID-19 vaccine. Additionally, current and upcoming research aspects have also been discussed.
ORIGIN AND NAMING
The world has witnessed three pandemic outbreaks by the members of the family ‘Coronaviridae’ in the past two decades. In November 2002, first Severe Acute Respiratory Syndrome (SARS) by SARS-Coronavirus (SARS-CoV) had been identified in Guangdong province, Southern China. SARS outbreak continued till July 2003 and there were 8098 confirmed and 774 deaths. The second outbreak, Middle East Respiratory Syndrome (MERS) by MERS-CoV, was first reported in a 60-year-old Saudi Arabian citizen in 2012. MERS was spread to 27 countries and there were confirmed cases and deaths were 2494 and 858, respectively (about 1 in 3). Asian civet cat (Paguma larvata) and dromedary camels were the animal reservoir of SERS and MERS, respectively which is shown in Figure 1. Recently, the last and deadliest outbreak by the betacoronavirus genus is the COVID-19 by a novel coronavirus where bat is suspected as the zoonotic reservoir. It was first identified in December 2019 in Wuhan, China and WHO declared it as a “Public Health Emergency of International Concern” on 1st February 2020 [15] [16]. Till the writing of this article, 215 countries, areas or territories have been affected with 6,999,124 confirmed cases and 402,564 confirmed deaths [3].
It is presumed that the natural host of SARS-CoV-2 may be the bat Rhinolophus affinis, as the genome nucleotide sequence of coronavirus detected from bat has 96.2% whole genome identity with 2019-nCoV [6]. However, the genetic distance suggests an intermediate in between, which lately presumed as Malayan pangolins. After analyzing 1000 metagenomic samples of pangolins, it was found that 70% of them contained β-CoV and some genome sequence shows maximum 99.92% similarity with the 2019-nCoV [17].
Initially, the newly recognized human pathogen was named as “2019-nCoV” by WHO on January 12, 2020. Later on, the Coronavirus Study Group (CSG) of the International Committee on Virus Taxonomy (ICVT) suggested the name as SARS-CoV-2 as the placement of 2019-nCoV on February 11, 2020 [18, 19].
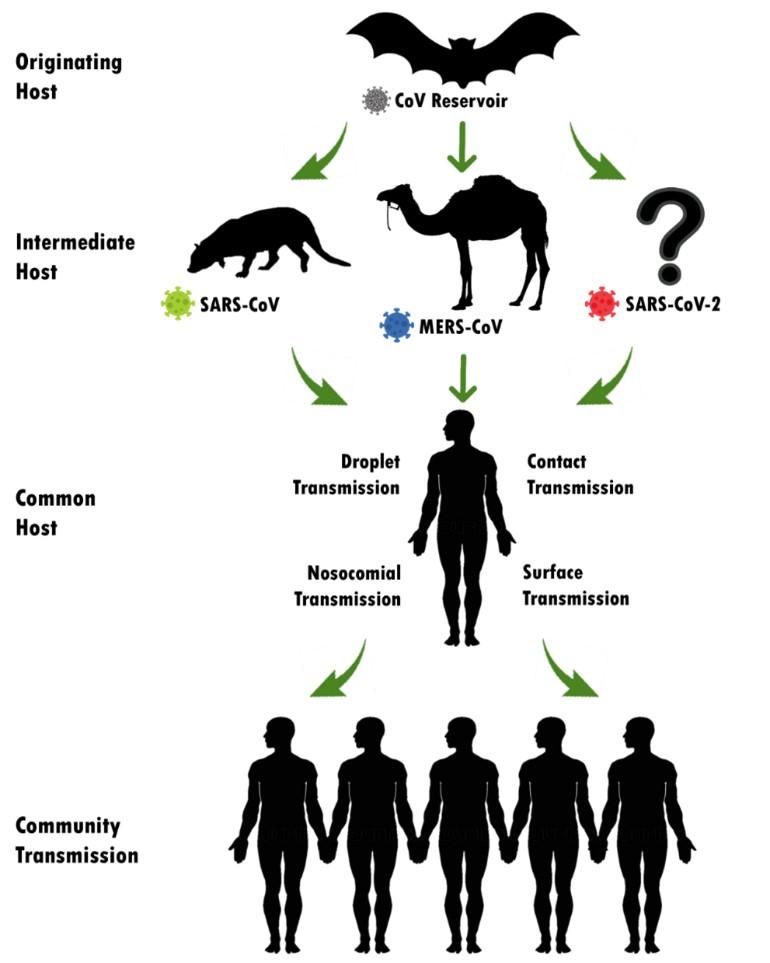
TRANSMISSION AND PATHOGENESIS
According to the latest guidelines from Chinese Health Authorities, SARS-CoV-2 generally transmits from person to person via 3 main routes: 1) droplets transmission, 2) contact transmission and 3) aerosol transmission [20]. Infection transmitting droplets varies into different sizes: respiratory droplets are >5-10 µm in diameter, where droplets ranged ≤5μm in diameter is named droplet nuclei. Droplet transmission occurs when a healthy individual is in close contact (1 meter) to COVID-19 affected persons and his/her mucosae (nose, mouth) or conjunctiva (eyes) get exposed to the infective respiratory droplets (via sneezing and/or coughing). In other way around, droplet nuclei are way lighter and stay in the air for longer periods and can transmit to others who are distant more than 1 meter, which should not be mistaken with airborne transmission. To date, no airborne transmission has yet been reported [8].
Direct or indirect contact happens when a subject touches an infected person, a surface or object contaminated by the virus and afterward touches his/ her mouth, nose or eyes which could transmit the virus [20]. Some studies suggested that, through specific medical setup and by procedure specificity, 2019-nCoV could be airborne through fomites and aerosol [8]. It is also noteworthy that, there is evidence of COVID-19 infection leading to intestinal infection and being present in feces [21]. In this case subjects’ nasopharyngeal testing result came negative, consecutive rectal swabs testing came positive all around, indicating the possibility of fecal-oral transmission [7]. However, the aerosol and fecal transmission routes still need to be further studied and confirmed before drawing public attention.
It should be noted down that though there had been a case report from The New England Journal of Medicine suggesting 2019-nCoV could be spread asymptomatically [22], Kai Kupferschmidt, a correspondent of Science magazine claimed the case report as flawed with significant proves [23].
After entering the host through a different portal of entries, Coronavirus (CoV) enters the host cells through binding cell surface receptor by its spike proteins for its replication and finally causing infection [24]. Angiotensin-converting enzyme 2(ACE2) is the spike protein of 2019-nCoV for the entry [25]. By exploiting the host cell machinery, viral proteins are translated from CoV positive-sense RNA, which further undergoes proteolysis mainly by two proteinases namely, coronavirus main proteinase (3CLpro) and the papain-like protease (PLpro) [26]. CoV replicates its genomic material by a replicase which is RNA-dependent RNA polymerase (RdRP) [27]. The above-mentioned spike, RdRP, PLpro and 3CLpro are the possible logical targets for formulating new therapeutics. Figure 2 illustrates the mechanism of pathogenicity and potential therapeutic development of SARS-CoV-2.
RISK FACTORS REGARDING COVID-19
Reports from the mainland China suggested that most of the confirmed cases were mild or moderate but some of the cases were severe (14%) and critical (5%) [28]. European centre for disease control (ECDC) stated that the COVID-19 risk is moderate for the general people [29] but very high for the older adults and persons who are suffering from chronic diseases [29] [30]. Older age, cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and cancer were all associated with an increased risk of death. Some studies suggested obesity and smoking can increase the risk of severe illness [31]. A study from China found that the deaths between 40-60 year was 16.8%, more likely be to male and having a comorbidity e.g. diabetes, cardiovascular disease, hypertension, or chronic lung disease while who survived were on average 17 years younger [32]. These results have similarities with many other cases in China [31]. Other risk factors could be lack of ICU, ventilator, sampling and testing materials which could accelerate mortality rate [29]. Healthcare personnel are also at risk as they must work in the clinic and hospital. Twenty percent of responding workers were infected in Italy and some of them died [33]. Some countries are even unable to ensure personal protective equipment (PPE) to the health workers and putting them at a great risk of infection which could lead to death [33].
SIGNS AND SYMPTOMS
The symptoms of COVID-19 are not specific which could be asymptomatic to death causing severe pneumonia. Centre for disease control and prevention (CDC) declared a wide range of symptoms include fever, cough, shortness of breath, chills, repeated shaking with chills, muscle pain, headache, sore throat and new loss of taste or smell. WHO suggested that the COVID-19 symptoms are usually mild and begin gradually [34]. From the previous knowledge of MERS-CoV virus’s incubation period, CDC suggests that the symptoms might take 2-14 days to appear after the exposure [9]. One of the initial studies of 41 patients in Wuhan, China by Chaolin et al. (2020) noted the most common symptoms of the COVID-19 disease i.e. fever (98%), cough (76%), myalgia or fatigue (44%). Some atypical symptoms included as sputum (28%), headache (8%), hemoptysis (5%) and diarrhea (3%). Dyspnea was observed in almost half of the patients and 63% of patients showed lymphocytopenia [35]. Lai et al. (2020) studied three relatively large-scale data of pneumonia patients (278) caused by SARS-CoV-2 in Wuhan [36]. Here, the most common symptoms were fever (92.8%) followed by cough (69.8%), dyspnea (34.5%), myalgia (27.7%), headache (7.2%) and diarrhea (6.1%). Furthermore, a small number of the patient had rhinorrhea (4.0%) and sore throat (5.1%) [36]. Another study by Wei-jie Guan and colleagues with 1099 laboratory-confirmed cases, stated that 43.8% of patients had fever on admission and 88.7% by the time of hospitalization and 67.8% had cough. Additionally, 83.7% of patients had lymphocytopenia and only 3.8% had diarrhea [37]. One recent study reported a new symptom for COVID-19 as the loss of taste and smell. The authors claimed that 30% of positive patients from South Korea experienced anosmia as primary symptom and there is evidence of anosmia in Germany and ageusia in Italy and Switzerland among COVID-19 patients [38]. Data from eight different research groups were accumulated in Table 1 for getting a clear prediction of the syndrome of COVID-19, where the most frequent symptoms are fever (81.44%) followed by cough (64.79%), dyspnea (31.6%), fatigue (28.71%), and myalgia (27.71%). Other mention-worthy signs and symptoms were headache, diarrhea, sputum production, vomiting and nausea, etc.
Table 1. Summarization of the signs and symptoms of COVID-19 in percentage based on the clinical investigations of eight research teams.
DIAGNOSIS OF COVID-19
Even if vaccines and other legit therapeutics establishments are underway, there is currently no Food and Drug Administration (FDA) approved vaccines available to conquer the pandemic. That’s why proper diagnosis in mass-scale could minimize the infestation through case identification, isolation and contact tracing [11]. The diagnosis can be indicated as two types based on their availability, result generation speed and reliability. Different serological assays like Rapid Diagnostic Test (RDT), Enzyme-linked Immunosorbent Assay (ELISA) and Neutralization Assay are vastly being used right now.
Compared to ELISA and Neutralization Assay which takes 1-5 h and 3-5 days, respectively, RDT is the most rapid immunoassay as it takes only 10-30 minutes to generate the result [45]. Zhengtu Li and his team developed a new immunoassay, point-of-care testing, which can detect IgM and IgG simultaneously to detect SARS-CoV-2 from the blood and serum samples within 15 minutes [46].
The test kit has shown some false-negative results as well. It’s hard to determine when the patient exactly gets infected and elevated IgM antibodies disappear after two weeks, which couldn’t meet the required peak of the IgM antibody. The variation of individual antibody production rate can be another reason for the false-negative result exhibition of COVID-19 patients [46].
Rather than immunoassay based diagnosis, different commercial and non-commercial serological testing methods still under development which may show even better and rapid viral detection [11]. Nevertheless, molecular testing using real-time Reverse Transcriptase Polymerase Chain Reaction (rRT-PCR) is the most accurate diagnostic procedure because of its ability to target or identify specific pathogens [11]. The samples are collected from the subject’s respiratory tracts. Though upper respiratory samples (nasopharyngeal and oropharyngeal swabs, nasal aspirates) are broadly recommended, lower respiratory samples (sputum, BAL fluid, and tracheal aspirates) are also tested when patients are showing negative test result from upper respiratory samples but exhibiting productive cough and all other COVID-19 symptoms [12]. RNA is extracted from the samples, which is converted to complementary DNA (cDNA) through the reverse transcription process, followed by specific region amplification through primers. The RdRP gene (RNA-dependent RNA polymerase gene) in the ORF1ab region, the E gene (Envelope protein gene) and the N gene (nucleocapsid protein gene) are discovered as the conserved sequence of SARS-CoV-2 and vastly in use as primers in the detection process [47]. The assay can then be configured as a two-target system where one primer is responsible for detecting numerous types of coronaviruses including SARS-CoV-2, whereas the other primer is specified only for SARS-CoV-2 detection. After optimizing the assay conditions by taking rigorous steps to set reagent condition, incubation times and temperatures, the PCR test takes place. To ensure the reliability of the test and to detect experimental failures, controls must be carefully chosen [11].
Though viral nucleic acid RT-PCR testing has become the current preferable diagnostic method for SARS-CoV-2 detection, there are some limitations as well. In this crucial time, this testing procedure requires hours to days to generate results, requires complicated laboratory setup, expensive equipment and trained technicians to execute the process. Aside from these, there had been some false-negative cases of RT-PCR generated results [46]. Because of the shortage and time consumption of RT-PCR, the Hubei Province, China used chest Computed Tomography (CT) scans temporarily for diagnosis, where many X-rays are taken from different angles from the patient’s chest to analyze cross-sectional images. The indications of COVID-19 infection include bilateral and peripheral ground-glass opacities (GGO) and consolidations of the lungs [36].
POTENTIAL DRUGS AND SUPPORTIVE ASPECTS
Although there are no approved drug therapies, common anti-malarial drugs Chloroquine (CQ) and Hydroxychloroquine (HCQ), developed in 1934, have previously gained considerable attention as a possible drug option for COVID-19. Chloroquine can inhibit the viral entry to the host cell by inhibiting viral binding to cell surface receptors and interfere with the viral replication process. Besides, this broad-spectrum antiviral drug can impede post-translational modification of viral proteins [48]. High concentration of Chloroquine analogs has exhibited anti-viral activity against SARS-CoV-2 (EC50=1.13 μM in Vero E6 cells), HIV, dengue, hepatitis C, chikungunya, influenza, Ebola, SARS and MERS viruses in vitro through inhibiting acidification of endosomes [49] [50]. In China, multicenter trials of Chloroquine phosphate against COVID-19 related pneumonia exhibited effectiveness and safety of this drug and is recommended for the treatment of COVID-19 associated pneumonia. More than ten hospitals in China were involved in these trials and results from more than 100 patients exhibited that this drug was successful in inhibiting the severity of pneumonia, relieving viruses and improving the outcome of lung scanning [51]. In April 2020, a trial on 62 COVID-19 patients with HCQ in the Renmin Hospital of Wuhan University was uploaded in the medRxiv server [52]. In this trial, 31 patients in the control group received standard treatment and 31 patients in HCQ treatment group received additional HCQ sulfate tablet (400mg/d) for five days. 80.6% of the HCQ treatment group exhibited improved pneumonia conditions compared to 54.8% of the control group. Also, four patients of the control group proceeded to severe illness, whereas no patient of HCQ treatment group confronted such a situation. In addition, body temperature recovery and cough attenuation period were significantly decreased in the HCQ treatment group compared to the control group [52]. Though the Food and Drug Administration (FDA) authorized temporary use of CQ and HCQ for the treatment of COVID-19 hospitalized patients, on April 24, 2020 FDA reported the cautions against the use of these two drugs due to the serious heart rhythm problems in COVID-19 patients [53]. The study conducted on 368 COVID-19 patients surprisingly supports the findings of FDA as either HCQ or HCQ with Azithromycin could not lower the breathing complications, but the patients treated with only HCQ elevated mortality [54]. Therefore, it needs further investigations regarding the protective roles of HCQ alone and combined with Azithromycin or other antibiotics and other therapeutic molecules. A recent study published in the Nature Cell Research showed Remdesivir was highly efficient in controlling 2019-nCoV in Vero E6 cells and in a human cell line [49]. In Washington, Remdesivir was administrated to a 35-year-old man, the first case of COVID-19 in the United States. Remdesivir exhibited promising outcomes in that case, however, controlled trials are required [21]. A randomized and controlled trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) revealed that Remdesivir treatment resulted in faster recovery of hospitalized advanced COVID-19 patients [55]. In particular, remdesivir treatment group exhibited 31% faster recovery (11 days) time than that of placebo group (15 days) [55]. However, in ten hospitals in Hubei, China, randomized, double-blind, placebo-controlled trial of remdesivir on COVID-19 patients didn’t show expected result. Among 155 remdesivir administrated patients, 102 (66%) exhibited adverse effect and in 18 patients (12%) were stopped early due to the unfavorable effects [56]. The status of pneumonia was surprisingly improved within 24 hours in remdesivir administered COVID-19 patients without specific side effects in USA [57,21]. Lopinavir showed in vitro inhibitory activity against SARS-CoV and MARS-CoV and a combination of lopinavir–ritonavir with ribavirin (a guanosine analogue) reduced the death risk in SARS patients. The actions of ribavirin plus both intereferon-α and ciclesonide, a glucocorticoid drug (prescribed for asthma) were also considered as SARS-CoV-2 therapeutic option [58].
An RdRp inhibitor, favipiravir was used to treat Ebola, emerging influenza in Japan and most recently COVID-19 in China [59, 60]. However, a clinical trial on 199 SARS-CoV-2 infected patients treated with lopinavir–ritonavir didn’t show any extra differences or benefits [61]. Furthermore, on February 14, 2020, a clinical trial of Favipiravir (RNA-dependent RNA polymerase inhibitor) against COVID-19 was initiated by the Clinical Medical Research Center of the National Infectious Diseases and the Third People’s Hospital of Shenzhen showed that this drug had more formidable anti SARS-CoV-2 activity than lopinavir/ritonavir without any remarkable adverse effect [59].
Moreover, Azithromycin and Tocilizumab can be prospective adjuvant therapy for COVID-19 [13]. An antibiotic, Teicoplanin prescribed for Methicillin-resistant Staphylococcus aureus (MRSA) which has been reported to be functional against COVID-19 [62]; [63]. The clinical trials of a wide range of therapeutics such as chloroquine/hydroxychloroquine, Bacillus Calmette- Guérin (BCG) vaccine, recombinant human interferon alpha-1b (rhIFNα) nasal drop, lopinavir/ritonavir, chloroquine plus azithromycin, imatinib, favipiravir, telmisartan, colchicine, aspirin, statin and dexamethasone conducted on thousand cases in many countries like as Spain, Canada, Ireland, South Africa, UK, USA, Zambia, China, Singapore, France, Australia, New Zealand, Nigeria, Pakistan and Brazil have got many primary findings but did not conclude absolute perfect one for COVID-19 treatment [64]. These clinical trial-based findings could not establish publicly accepted protocol as well. The effective antiviral impacts of most the tested drugs at extensive level against SARS, MERS or SARS-CoV-2 were observed or the tested drugs were shown to act as the immunomodulatory actions which are believed to decreases the severe lung inflammation at later stages of COVID-19 infection [65-67]. Co-administration of darunavir and umifenovir showed anti-SARS-CoV-2 effects in patient therapies to whom intensive cares were also provided [66]. These findings stressed the scientific communities for designing other more specific antiviral therapeutics for COVID-19 and also pushed the health care providers for exclusive supports for COVID-19 patients.
It is reported that the nutritional aspects, together with drug interventions not only mitigate CoV infections but also boost up the immunity of the patients. Vitamins A, B, C, D and E, omega‐3 polyunsaturated fatty acids (PUFA) as well as some metals such as selenium, zinc and iron have been reported to possess protective roles against Coronaviruses [68]. Therefore, the single or combined use of them might be very effective nutritional support to combat against SARS-CoV-2. Also, the immune-enhancers, for example, interferons, intravenous gamma globulin, thymosin α‐1, thymopentin, levamisole, cyclosporine A and Chinese medicine exhibited inhibitory activity against SARS‐CoV, MERS‐CoV and avian infectious bronchitis viruses [68]. Potential therapeutic development sites against SARS-CoV-2 have been illustrated in the Figure 2.
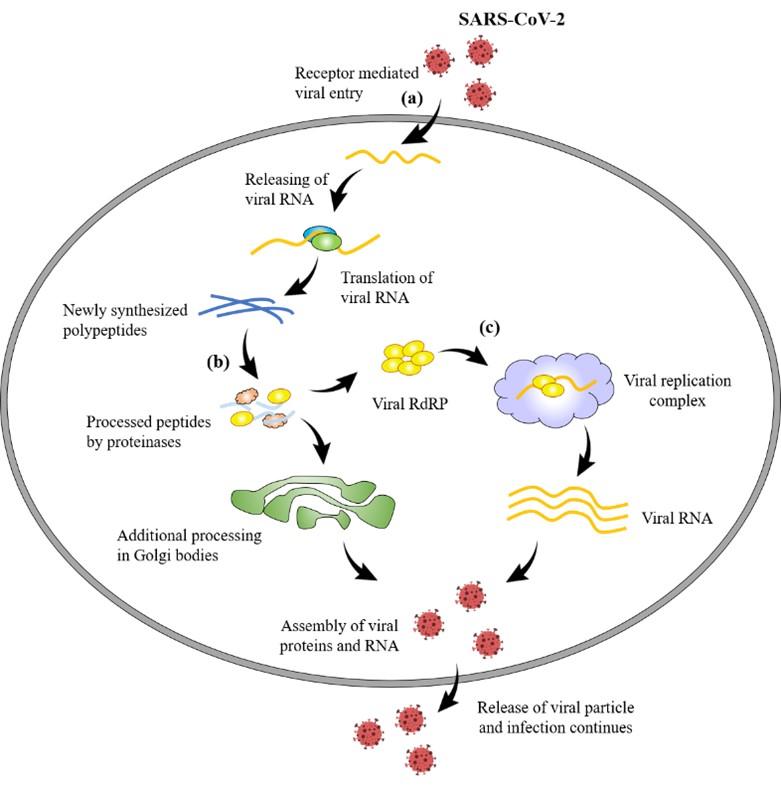
CHALLENGES AND PROSPECTS TO DEVELOP COVID-19 VACCINE
Challenges
In this current global outbreak of COVID-19, a potential vaccine is a crying need to prevent and combat the disease. Developing a vaccine for infectious disease is usually very time consuming and requires enormous financial support [70]. Safety is the primary concern in developing drugs or vaccines, so it is not recommended to rush in deploying COVID-19 vaccines without maintaining proper safety measures [71]. There are some challenges in developing the vaccine against COVID-19. For instance, Tang et al. (2020) analyzed genomes of SARS-CoV-2 from 103 patients in China and claimed for finding differences in the genome [72], which added a new concern in vaccine development as we might require different vaccines for different genomes. Also, SARS and MERS vaccine candidates intensified the lung disease directly or through antibody-dependent augmentation at preclinical trials. Moreover, to ensure the highest immune response, the most important and critical part is the optimization of antigen design and there is an argument that whether targeting the whole viral protein or just the receptor-binding domain will be the most suitable approach [73]. Besides, a wide range of non-approved drugs for SARS-CoV-2 are being applied to the infected patients though some were effective some were not. As a result, SARS-CoV-2 is getting exposed to different therapeutic compounds which are providing the opportunities to SARS-CoV-2 to be mutated and to be more virulent to human. Changes in the genetic materials might alter the currently available therapeutic targets such as spike protein, RdRP, the main two proteinases PLpro and 3CLpro of SARS-CoV-2. Early studies reported severe illness of SARS-CoV-2 at more than 60 years [74] but recently severe cases have been found in young age groups too, this might be the result of infection of new SARS-CoV-2 strain emerged due to mutation or other reasonable reasons. Besides, the natures of SARS-CoV-2 have not been studied to clearly in response to geographical location and environment too. Moreover, due to the lack of enough data, it is difficult to conclude that people of which age groups are more susceptible to SARS-CoV-2 infection as SARS-CoV and MERS-CoV are strongly related to host conditions including age, biological sex, and overall health [75].
Prospects
However, according to the landscape of COVID-19 candidate vaccines by WHO, until June 2, 2020, there are ten candidate vaccines at clinical evaluation and 123 vaccine candidates are in preclinical evaluation [14]. The University of Oxford and AstraZeneca researchers are the first to begin phase 3 trial of their non-replicating viral vector vaccine and expecting the outcome by summer, 2020. The scientists are engineering a chimpanzee adenovirus to carry DNA for the spike antigen [76]. Other groups using non-replicating viral vector platform are CanSino Biological Inc and the Beijing Institute of Biotechnology, currently in phase 2 of their clinical trial. RNA (mRNA) based approaches reached to clinical trials by Moderna and National Institute of Allergy and Infectious Diseases (phase 2) and BioNTech, Fosun Pharma and Pfizer (phase 1/2) [14]. Similar to maximum COVID-19 vaccines development approaches, Moderna’s candidate are attempting for training the immune system in order to recognising SARS-CoV-2’s spike protein [76]. Moreover, inactivated vaccines from Wuhan Institute of Biological Products/Sinopharm, Beijing Institute of Biological Products/Sinopharm and Sinovac are in the phase 1/2 clinical evaluation. Clinical trial of a protein subunit vaccine (phase 1/2) and a DNA vaccine (phase 1) are conducting by Novavax and Inovio Pharmaceuticals respectively. Furthermore, Institute of Medical Biology of Chinese Academy of Medical Sciences is in phase 1 of their inactivated vaccines’ clinical evaluation [14].
Among the preclinical vaccines of COVID-19, approaches based on protein subunit are around one third of total evaluation. Sanofi and GlaxoSmithKline, University of Queensland, University of Alberta and some other teams throughout the world are working on protein subunit approach. Live attenuated vaccine is developing by the Serum Institute of India/ Codagenix and Indian Immunologicals Ltd/Griffith University. Furthermore, some companies are working on nucleic acid-based vaccines, for example Inovio Pharmaceuticals (DNA based), Curevac (mRNA based). Live attenuated vaccine is developing by the Serum Institute of India/Codagenix and Indian Immunologicals Ltd/Griffith University. Furthermore, some companies are working on nucleic acid based vaccines, for example Inovio Pharmaceuticals (DNA based), Curevac (mRNA based) [77]. The development of viral vector vaccines against SARS-CoV-2 is in progress by Tonix Pharma and Janseen Pharmaceuticals by adapting the Horepox virus and AdVac® adenoviral vector platform, respectively [78]. The updated list of vaccines against COVID-19 is available at the Draft Landscape of COVID-19 candidate vaccines by WHO.
UPCOMING RESEARCH TO COMBAT COVID-19
In course of time much more data are getting available to case vitality rates, transmissibility, and overall natural history of COVID-19 infection [79]. Considering the potential warning of the pandemic COVID-19 infections, researchers and physicians are trying their best to understand the new pathophysiology of this novel coronavirus to invent probable treatment strategies, successful therapeutic agents and vaccines [80]. It can be highlighted that antiviral strategies related to small molecules and biologics focusing on complex molecular interactions engaged with coronavirus infection and replication. The drug-repurposing endeavor reported herein emphasized predominantly on the agents proved to be compelling against SARS-CoV, MERS-CoV and other RNA viruses [81].
The present investigation of coronavirus related biologics such as therapeutic antibodies, cytokines, and nucleic acid-based therapies specially focused on virus gene expression and different types of vaccines [80]. The raised level of inflammatory cytokines IL-6, IL-2, IL-1β, IL-8, IL-17, IFN-γ, TNF-α, IP10, MCP-1, IL-10 and IL-4 resulted cytokine release syndrome (CRS) which might have influential functions in the pathophysiology of COVID-19 [66]. This cytokine aspect should be considered by the scientific societies for the novel therapeutic development of COVID-19. Another important consideration is reinfection of SARS-CoV-2 which is related to cellular immunity and neutralizing antibodies. Hence designed anti-SARS-CoV-2 vaccines should induce rigid cellular immunity and raise the required level of titer of the neutralizing antibodies to make sure no reinfection in the vaccinated populations [82]. Therefore, it is necessary to formulate enzyme-linked immunosorbent assay (ELISA) that enables to quantify anti-receptor binding domain (anti-RBD) antibodies and its correlation with neutralizing antibodies. To generate SARS-CoV specific and neutralizing human monoclonal antibodies (hmAbs), several methods have been applied, for instance, transgenic mice immunization, small chain variable regions’ cloning from immature and convalescent patients as well as convalescent B cells’ immortalization [83].
A study on 129 COVID-19 confirmed cases at mild stage in Wuhan Union Hospital of China suggested the antiviral combined therapies at early stage of infection as it took 7 days less to eradicate viruses compared to late stage of infected cases [84]. Zuo et al. recently demonstrated the shortened SAR-CoV-2 shedding period by administering combined actions of lopinavir/ritonavir plus IFN-α [85]. So, it is another window to carry on research for the development of drugs/vaccines considering the patient physiological situations at early stage of COVID-19 infections. In addition, low dose radiation (1.5 Gy) to COVID-19 pneumonia patient’s both lungs for 10-15 minutes resulted in the improved breathing and recovery on an average 1.5 days [86].
In current situation, virophage (virus eater) might be a potential research option to destroy this type of notorious virus. It is mention-worthy, because of the emergence of multidrug-resistant bacterial infections, phage therapy has undergone a renaissance [87]. Virophages are double-stranded DNA virus that can infect other viruses and Sputnik was the first isolated virophage which was isolated in 2008 [88,89]. Though virophage has a host range for large-size viruses, future research may isolate prospective virophage with a host range of SARS-CoV-2.
IMPACT OF COVID-19 ON GLOBAL ECONOMY
The pandemic is playing a devastating role to push the global economy to the verge of great threat, affecting all aspects of economy. According to the United Nations Conference on Trade and Development (UNCTAD) reports, Covid-19 is likely to cost the global economy a minimum of $2 trillion in 2020 [90]. China, where the SARS-Cov-2 virus originated in, is the world’s most populous country and the world’s second biggest economy with a GDP of $13.6 trillion. The country had been fighting the pandemic since December 2019 and taking a great toll in Gross Domestic Product (GDP) which fall to 4.9% in 2020, compared to 6.1% in 2019. The Organisation for Economic Co-operation and Development (OECD) announced that growth prospect for United States of America (USA) the world’s biggest economy, lowered to 1.9% this year after 2.3% in 2019. Consequently, global economic growth is predicted to decline to 2.4% in 2020, compared to 2.9% in 2019 [91].
According to the World Travel & Tourism Council (WTTC), tourism is a significant global sector contributing 10.4% of global GDP and 10% of global employments [92]. With the global spread of the virus, the World Tourism Organization (UNWTO) has reported that an estimated downturn in foreign tourist arrivals will be between 20% and 30%, which could result in a drop in international tourism receipts of between 300-450 billion dollars [93]. In view of the deteriorated airline industry, the International Air Transport Association (IATA) estimated losses in the amount of $252 billion and a 44% decrease in lost revenues [94].
General Administration of Customs acknowledged that China, ranked first in goods and services exportation, falls overall exports and imports by 17.2% and 4%, respectively in the first two months of 2020. During that time, industrial production in China dropped by 13.5%, while industrial profit dropped significantly by 38.3% [92]. The International Labour Organization (ILO) has disclosed that the global economic downturn triggered by COVID-19 will eliminate 5.3 to 24.7 million jobs, which means by the end of 2020, a major drop of earnings between $860 billion to $3.4 trillion will happen for the workers [95]. In March 2020, as an example, the unemployment rate in the USA rose by 0.9% to 4.4% [92]. This situation will, in the long term, lead to a deterioration of human assets that has a great effect on countries’ economic development.
CONCLUSIONS
This study shows not only a universal scenario of the occurrence, distribution, death toll and the aftermath of the current pandemic due to COVID-19 but also the scenario of biomedical research to develop medicine/vaccine. Initially, a lot of studies have been explored based on etiological, epidemiological and diagnostic research. Now research on prevention and control measures have been increasing gradually. Potential studies in this dominion are crying need to control the pandemic soonest possible and to minimize the death toll. The public-private partnership, as well as multinational collaborative research, is essential to eradicate the virus SARS-CoV-2 from the universe like smallpox. This study also recommends the global scientific community and the policymakers for provisioning short-term and long-term public health protection measures to cope up with such type of public health emergency.
ACKNOWLEDGEMENT
We want to acknowledge and grateful to Daniel TA (School of Science, Western Sydney University, Australia) for manuscript language editing service.
CONFLICT OF INTEREST
The authors do not declare any conflict of interest.
AUTHOR CONTRIBUTIONS
SKB conceived the idea and supervised the project. MMS and SBS performed the database search and literature reviews. SKB, MMS, SBS, SHKB, MRH, RI and DKP wrote the manuscript. SHKB and SBS illustrated the figures. SBS and MMS prepared the table. SKB, DKP and RI critically revised the manuscript. All authors proofread and approved the final manuscript.
References
- [1]Kinross P, Suetens C, Gomes Dias J, Alexakis L, Wijermans A, Colzani E, et al. Rapidly increasing cumulative incidence of coronavirus disease (COVID-19) in the European Union/European Economic Area and the United Kingdom, 1 January to 15 March 2020. Eurosurveillance 2020; 25:1–5.
- [2]WHO. Coronavirus disease 2019 (COVID-19) Situation Report – 77. 2020.
- [3]Covid-19 Coronavirus Pandemic. (2020). WorldOmeter. Retrieved June 7, 2020, retrived from https://www.worldometers.info/coronavirus/
- [4]Ranney ML, Griffeth V, Jha AK. Critical Supply Shortages — The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N Engl J Med 2020 (382:e41); 5–7.
- [5]European center for disease prevention and control. (2020). Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – seventh update, 2019 (March).
- [6]Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020;12:1–6.
- [7]Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020;26:502-505.
- [8]WHO. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. 2020.
- [9]Symptoms of Coronavirus. (2020). Centers for Disease Control and Prevention (CDC). Retrieved April 8, 2020, from https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
- [10]Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. J Am Med Assoc 2020;323:1407.
- [11]Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC, et al. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020; 14: 3822–3835
- [12]WHO. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Interim Guid 2020:1–7.
- [13]Smith T, Bushek J, Prosser T. COVID-19 Drug Therapy – Potential Options. Clin Solut 2020.
- [14]World Health Organization. Draft landscape of COVID-19 candidate vaccines – 2 June, 2020.
- [15]Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol 2020:1–10.
- [16]Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis 2020; e102–07
- [17]Lam TT-Y, Shum MH-H, Zhu H-C, Tong Y-G, Ni X-B, Liao Y-S, et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature 2020.
- [18]Jiang S, Shi Z, Shu Y, Song J, Gao GF, Tan W, et al. A distinct name is needed for the new coronavirus. Lancet 2020;395:949.
- [19]Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet 2020;395:470–473.
- [20]Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect Dis Poverty 2020;9:1–12.
- [21]Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36.
- [22]Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med 2020;382
- [23]Kupferschmidt K. Study claiming new coronavirus can be transmitted by people without symptoms was flawed. Science 2020.
- [24]Li F, Li W, Farzan M, Harrison SC. Structural biology: Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005;309:1864–1868.
- [25]Dong N, Yang X, Ye L, Chen K, Chan EW-C, Chen S. Genomic and protein structure modelling analysis depicts the origin and pathogenicity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. F1000Research 2020;9:121.
- [26]Ziebuhr J, Snijder EJ, Gorbalenya AE. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J Gen Virol 2000;81:853–79.
- [27]Xu X, Liu Y, Weiss S, Arnold E, Sarafianos SG, Ding J. Molecular model of SARS coronavirus polymerase: Implications for biochemical functions and drug design. Nucleic Acids Res 2003;31:7117–30.
- [28]Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA – J Am Med Assoc 2020; 323:1239-1242.
- [29]ECDC. Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – seventh update. 2020.
- [30]Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395: 1054-1062.
- [31]Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. Bmj 2020;1198:m1198.
- [32]Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj 2020;1091:m1091. h
- [33]Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet 2020;2:10–3.
- [34]Coronavirus- Symptoms. (2020). World Health Organization (WHO). Retrieved April 8, 2020, from https://www.who.int/health-topics/coronavirus#tab=tab_3
- [35]Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis 2020; 94: 44-48.
- [36]Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 2020;55:105924.
- [37]Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv 2020.
- [38]Gautier J-F, Ravussin Y. A New Symptom of COVID-19: Loss of Taste and Smell. Obesity 2020; 28.
- [39]Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA – J Am Med Assoc 2020;323:1061–9.
- [40]Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13.
- [41]Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 novel coronavirus (2019-NCoV) pneumonia. Radiology 2020;295:210–7.
- [42]Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506.
- [43]Lei C, Huiguo L, Wei L, Jing L, Kui L, Jin S, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chinese J Tuberc Respir Dis 2020; 43:E005.
- [44]Chang D, Lin M, Wei L, Xie L, Zhu G, Cruz CS Dela, et al. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA – J Am Med Assoc 2020;323:1093.
- [45]Serology-based tests for COVID-19. (2020). Center for Health Security, John Hopkins University. Retrieved April 8, 2020, retrived from http://www.centerforhealthsecurity.org/resources/COVID-19/Serology-based-tests-for-COVID-19.html
- [46]Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and Clinical Application of A Rapid IgM‐IgG Combined Antibody Test for SARS‐CoV‐2 Infection Diagnosis. J Med Virol 2020;1–7.
- [47]Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25:1–8.
- [48]Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents 2020:105938.
- [49]Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71.
- [50]Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect 2017;5:1–13.
- [51]Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020;14:72–73.
- [52]Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv 2020; https://doi.org/10.1101/2020.03.22.20040758.
- [53]FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. Food Drug Adm 2020.
- [54]Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. MedRxiv 2020.https://doi.org/10.1101/2020.04.16.20065920.
- [55]NIH clinical trial shows Remdesivir accelerates recovery from advanced COVID-19. (n.d.). National Institute of Health. Retrieved June 7, 2020, from https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19
- [56]Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;0:1569–78.
- [57]Ko W, Rolain J, Lee N, Chen P, Huang C. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. J Antimicrob Agents 2020;55: 105933
- [58]Development of coronavirus treatment advancing in Japan with existing meds. (n.d.). The Mainichi. Retrieved June 6, 2020, retrived from https://mainichi.jp/english/articles/20200317/p2a/00m/0na/026000c
- [59]Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 2020;14:58–60..
- [60]Nagata T, Lefor AK, Hasegawa M, Ishii M. Favipiravir: A New Medication for the Ebola Virus Disease Pandemic. Disaster Med Public Health Prep 2015;9:79–81.
- [61]Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020:1–13.
- [62]Ramos-Martín V, Johnson A, McEntee L, Farrington N, Padmore K, Cojutti P, et al. Pharmacodynamics of teicoplanin against MRSA. J Antimicrob Chemother 2017;72:3382–9.
- [63]Baron SA, Devaux C, Colson P, Raoult D, Rolain JM. Teicoplanin: an alternative drug for the treatment of COVID-19? Int J Antimicrob Agents 2020;55:105944.
- [64]Davis JS, Ferreira D, Denholm JT, Tong SY. Clinical trials for the prevention and treatment of Coronavirus Disease 2019 (COVID-19): The current state of play. Med J Aust 2020.
- [65]Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;2:1–25.
- [66]Costanzo M, De Giglio MAR, Roviello GN. SARS CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and Other Drugs for the Treatment of the New Coronavirus. Curr Med Chem 2020;27.
- [67]Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun 2020;111:102452.
- [68]Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol 2020;92:479–90..
- [69]Wang C, Li W, Drabek D, Okba NMA, Haperen R van, Osterhaus ADME, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. BioRxiv 2020.
- [70]Ahn D-G, Shin H-J, Kim M-H, Lee S, Kim H-S, Myoung J, et al. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J Microbiol Biotechnol 2020;30:313–24.
- [71]Shibo Jiang. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature 2020.
- [72]Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev 2020; 0:1-12.
- [73]Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med 2020:1–5.
- [74]Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses 2020;12:1–8.
- [75]Fehr AR, Channappanavar R, Perlman S. Middle East Respiratory Syndrome: Emergence of a Pathogenic Human Coronavirus. Annu Rev Med 2017;68:387–99.
- [76]Mullard A. World Report COVID-19 vaccine development pipeline gears up. Lancet 2020;395:1751–2..
- [77]Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science 2020;368:948–50.
- [78]Zhang J, Zeng H, Gu J, Li H, Zheng L, Zou Q. Progress and Prospects on Vaccine Development against SARS-CoV-2. Vaccines 2020, Vol 8, Page 153 2020;8.
- [79]Quinn JM, Haggenmiller C, Wilson JM, McNamara T, Goebbels S, Hansen J-C, et al. Conference Report: Global Health Security Alliance (GLoHSA), a Product of the World Health Summit. Disaster Med Public Health Prep 2019:1–3.
- [80]Liu C, Zhou Q, Li Y, Garner L V., Watkins SP, Carter LJ, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent Sci 2020;6:315-331.
- [81]Gorbalenya AE, Baker SC, Baric RS, Groot RJ De, Gulyaeva AA, Haagmans BL, et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. BioRxiv 2020.
- [82]Manners C, Bautista EL, Sidoti H, Lopez OJ. Protective Adaptive Immunity Against Severe Acute Respiratory Syndrome Coronaviruses 2 (SARS-CoV-2) and Implications for Vaccines. Cureus 2020;2:6–13.
- [83]Coughlin MM, Prabhakar BS. Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Rev Med Virol 2012;22:2–17.
- [84]Yu T, Tian C, Chu S, Zhou H, Zhang Z, Luo S, et al. COVID-19 patients benefit from early antiviral treatment: a comparative, retrospective study. J Med Virol 2020.
- [85]Zuo Y, Liu Y, Zhong Q, Zhang K, Xu Y, Wang Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: a retrospective study in two designated hospitals in Anhui, China. J Med Virol 2020.
- [86]Hess C. B., Buchwald Z. S., Stokes W., Switchenko J. M., Nasti T. H., Weinberg, B. D., Khan M. K. (2020). Low-Dose Whole-Lung Radiation for COVID-19 Pneumonia: Planned Day-7 Interim Analysis of an Ongoing Clinical Trial. medRxiv 2020.
- [87]Oliveira H, Sillankorva S, Merabishvili M, Kluskens LD, Azeredo J. Unexploited opportunities for phage therapy. Front Pharmacol 2015;6:1–4.
- [88]Sobhy H. Virophages and their interactions with giant viruses and host cells. Proteomes 2018;6.
- [89]La Scola B, Desnues C, Pagnier I, Robert C, Barrassi L, Fournous G, et al. The virophage as a unique parasite of the giant mimivirus. Nature 2008;455:100–4.
- [90]The Coronavirus Shock: A Story of Another Global Crisis Foretold and Policymaker Should Be Doing About It. (n.d.). Geneva, Switzerland.
- [91]OECD. Coronavirus: The world economy at risk. OECD Interim Econ Assess 2020:1–15.
- [92]Açikgöz Ö, Günay A. The early impact of the Covid-19 pandemic on the global and Turkish economy. Turkish J Med Sci 2020;50:520–6.
- [93]International Tourist Arrival Could Fall By 20-30% In 2020. World Tour Organ 2020.
- [94]IATA Economics. COVID-19 Cash burn analysis. IATA Econ 2020.
- [95]International Labor Organization. COVID-19 and the world of work: Impact and policy responses 2020:1–15.