Optimization of surface sterilization method for the isolation of endophytic fungi associated with Curcuma longa L. and their antibacterial activity
Abstract
Medicinal plants have been extensively studied since ancient times and exploited for their various therapeutic applications. These plants have a repository of numerous beneficial chemical constituents imputable to their possible bioactive metabolites. Throughout the ages, turmeric (Curcuma longa L.) has a renowned nutritional and therapeutic importance. Each part of turmeric like rhizome, roots, stems, and leaves has its own medicinal properties. Inorder to recover endophytic mycoflora, epiphytic mycoflora must be eliminated via different sterilization techniques. In the present study, different sterilization methods have been assessed to isolate fungal endophytes from different parts of Curcuma longa L. The method involving the sequential washing of plant tissues with ethanol, sodium hypochlorite and ethanol used found more proficient in eliminating epiphytes. Five different surface sterilization treatments were evaluated for rhizome, roots, stem, and leaves of turmeric plant. Moreover, treatment 5 with combination of 70% ethanol for 1 min., followed by 2% sodium chloride for 2 to 3 min (2 min for leaf, and stem; 3 min for roots and rhizome) and 70 % ethanol for 30 seconds was found effective. A total of 38 fungal endophytes were recovered and screened for antibacterial activity against human pathogens Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa and Escherichia coli. Out of 38 isolates, only 11 (28.9%) isolates were found effective in inhibiting either one or all test pathogens. The results confirmed that turmeric plant of Haridwar region represents an extremely rich reservoir of potential endophytic mycoflora with possible source of novel bioactive metabolites. Further studies will resolute the potential of endophytic fungi for various biological activities such as antioxidant, antidiabetic and immunomodulatory.
INTRODUCTION
Curcuma longa (Turmeric) also known as Haldi, is a traditional Indian medicinal plant, a perennial member of the Zingiberaceae family. It is believed that the plant originated from Southeast Asia and is widely distributed among tropical and subtropical regions [1]. The main constituent of turmeric includes the curcuminoids, i.e., curcumin, demethoxycurcumin, and bisdemethoxycurcumin. The yellow color of turmeric is mainly due to the presence of curcumin in rhizome. Curcumin is a phenolic compound derived from the phenyl alanine and constitutes the major portion of food and nutraceutical. Curcumin has been reported to possess various biological activities such as antimicrobial, antioxidant, antidiabetic, anti-inflammatory and anticancer [2]. Turmeric is a golden spice cultivated in India since ancient times. Turmeric also possesses a variety of medicinal properties, including anti-inflammatory, antioxidant, hepatoprotective, antimalarial and anticancer, antidiabetic, antimicrobial, antidepressants, cardiovascular diseases, gastrointestinal disorders and anti-inflammatory [3-7]. Traditional medicinal plants are the treasure house for isolating endophytic species including bacteria, fungi, and actinomycetes, which can produce essential secondary metabolites [8, 9]. Within a plant, there is an existence of endophytic mycoflora, which robust within the healthy tissues, asymptomatically and assisting their host in a number of ways such as plant growth promotion, resistance against pathogens and production of secondary metabolites. Simultaneously, there has been a continual search for the discovery of novel bioactive compounds from endophytic mycoflora. Turmeric has been associated with a myriad of endophytic bacteria or endophytic fungi which protect the plant against biotic (defense against herbivory, allelopathic changes, protection from pathogens) and abiotic stresses (drought tolerance, salinity, temperature stress, heavy metal tolerance) and establishing host-plant symbiosis by supplying nutrients to the host [1].
Endophytes may penetrate the host plants either horizontally or vertically. Endophytic fungi compete with epiphytes and plant pathogens through the secretion of bioactive secondary metabolites. Thus, effective colonization helps in the regulation of a balanced host-endophyte relationship. Endophytic fungi also influence the host plants plant’s ability to fight disease caused by parasitic nematodes by several means, such as antibiosis, secretion of extracellular hydrolytic enzymes, parasitism, and hormones that promote plant defense mechanisms [10].
There is numerous research available for the isolation of endophytic fungi from different plant tissues [11]. The recovery of the endophytic microbiome usually depends on the sterilant used for surface sterilization method for the removal of epiphytes. The isolation of endophytes also depends on various factors like age of the plant, type of plant tissue, surface sterilant concentration, and time of exposure [12]. Surface sterilization is the most critical and threshold stage for recovering the fungal endophytes from inter/intracellular niches from the host plant, concomitantly eliminating the surface epiphytes [11, 14]. The most common chemical sterilant include ethanol, sodium hypochlorite, formaldehyde, and mercuric chloride [13]. But formaldehyde and mercuric chloride are excluded from our study due to their adverse deleterious effects. Also increased exposure time for surface sterilization may damage the plant tissue and hamper the recovery of endophytes [12]. The process of sterilization is difficult to carry out because the conditions required to kill the last bacterium or fungal spore on the surface may be lethal to some endophytic mycoflora as the sterilizing agent may penetrate the host tissue. Furthermore, a single sterilizing agent is ineffective to recover true fungal endophytes. Moreover, the use of different media for recovery of endophytic fungi resulting in the increased diversity of isolated fungi.
Only a few plants have been thoroughly investigated in terms of their endophytic mycoflora. There is a need to discover novel and beneficial endophytic microbes among the diverse range of plants found in various ecosystems. Studies on endophytic fungi from C. longa mostly focused on inhibitory activity [15], plant growth promotion, antagonistic and biocontrol agents [16, 17]. To the best of our knowledge, this is the first report on isolation of endophytic fungi from C. longa from Haridwar region of Uttarakhand, India which is in the foothills of the Himalayas, Uttarakhand and a typical low-lying territory flooded with water, valley, basin, marshy ground, marsh, swamp, and meadow. Endophytes acclimatized to such a unique environment are more likely to create novel bioactive metabolites. Therefore, our aim of present research work was to carry out to the influence of different sterilizing agents on isolation of endophytic fungi from rhizome, roots, stem and leaves of C. longa and their antimicrobial activity against human pathogens.
MATERIALS AND METHODS
Chemicals
Potato Dextrose Agar (PDA), Rose Bengal Agar (RBA), Water Agar (WA), Muller Hinton Agar (MHA) and Lactophenol cotton blue stain were obtained from HiMedia (Mumbai, India). Ethanol and sodium hypochlorite were procured from Sigma Chemical Company Ltd. (Aldrich, USA).
Collection of plant material
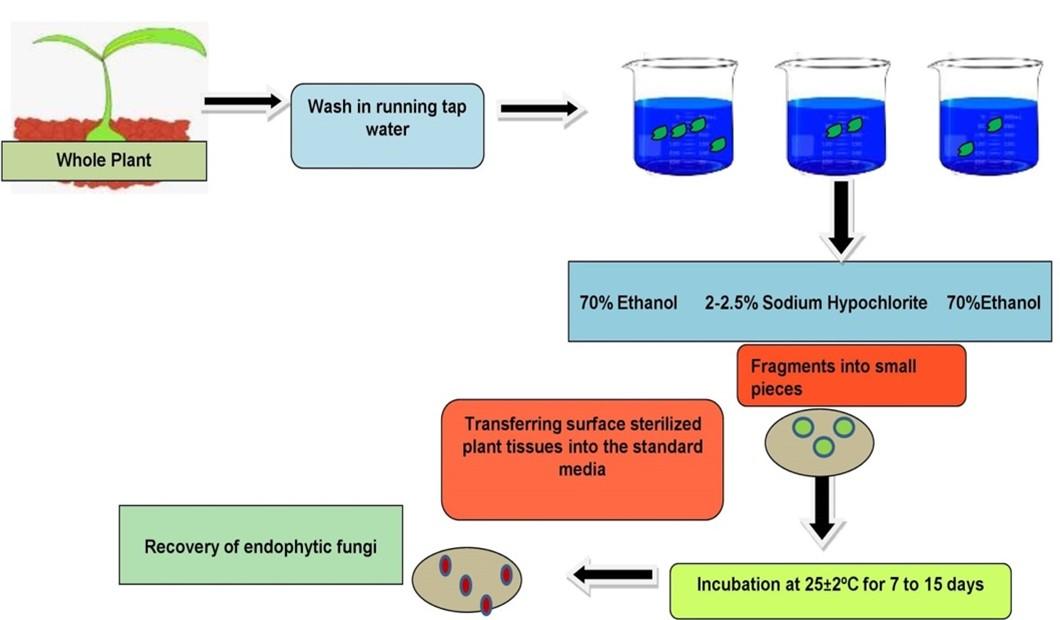
Rhizome, roots, stem, and leaves of healthy Curcuma longa L. plants were collected from the Haridwar region (latitude and longitude: 29.9457° N, 78.1642° E) of Uttarakhand, India in the month of July 2020 to December 2020. Three different sites were selected for isolation of fungal endophytes, i.e., Bahadrabad, Panjanhedi and Gurukula. The plant material was brought to the laboratory in sterile polyethylene bags (Himedia) and processed within a few hours after collection. Healthy and fresh plants were selected for the isolation of endophytic fungi. Explants that showed signs of physical damage or pathogenic infection were excluded from the study (Figure 1).

Isolation of endophytic fungi
Endophytic fungi were isolated from Curcuma longa using a standard protocol with minor modifications [17]. The most frequent method for isolating fungal endophytes is to surface sterilize the plant parts before plating it into the desired medium. The following stages were involved in the isolation and purification of endophytic fungi from plant tissue:
Pre-treatment
Pre-treatment includes washing of plant parts separately under running tap water for 10 to 15 minutes to remove dirt and debris from their superficial surface, followed by washing with sterile distilled water [18].
Surface sterilization
Sodium hypochlorite and ethanol were utilized as sterilizing agents, either alone or in combination, at various concentrations, to evaluate the surface sterilization process. Surface sterilization was done by sequentially rinsing the plant materials (rhizome, roots, stem and leaves) with different sterilizing agent mentioned in Table 1 for a specific period of time and followed by washing with sterile distilled water 2 to 3 times. Plant materials then dried in between folds of sterile filter paper under aseptic conditions.
Table 1. List of treatments used for the sterilization.
Efficiency of surface sterilization
There are four different methods to access the sterility check of surface sterilized plant tissues. Firstly, by imprinting the surface-sterilized tissue onto the potato dextrose agar, culturing of aliquots of the last wash solution onto PDA medium, dipping of the surface sterilized plant tissue in sterile distilled water and potato dextrose broth to observe the viability of microorganisms [11, 19] and finally by touching of the surface sterilized plant tissue with transparent cello adhesive tape and placing it on the lactophenol cotton blue stained slide.
Media for isolation of endophytic fungi
The choice of growth medium is essential as it directly influences the number of cultivable endophytic fungi isolated from plant tissues. For isolating endophytic fungi, standard media were used such as PDA, RBA, and WA. The growth medium was supplemented with standard antibiotic, chloramphenicol to avoid the growth of bacteria.
Isolation and identification of endophytic fungi
The surface sterilized plant tissues were cut into 0.5 x 0.5 cm pieces aseptically transferred onto the petri dishes with sterile forceps containing PDA, RBA and WA supplemented with antibiotics. Plates containing explants were sealed with parafilm and incubated at 25 ± 2°C for 7 days. The plates were observed for the formation of fungal colonies. The hyphal tips from the growing edges of the treated explants were picked with sterile needle and transferred aseptically onto a fresh PDA medium devoid of antibiotic. The isolated endophytic fungi were identified on the basis of their morphological characteristics such as fungal growth topography, colony colour, spore generation, growth rate, and colony margin with the help of lactophenol cotton blue staining [20, 21]. The isolated endophytic fungi were identified using the cello tape scotch method [22] and examined under a microscope at a magnification of 40 X. The pure cultures were maintained on PDA medium for further study.
Screening for antibacterial activity by agar disc diffusion assay
The antibacterial activity of isolated endophytic fungi was carried out using agar disc diffusion method for the rapid selection of bioactive microorganisms [23]. Two gram-positive bacteria: Staphylococcus aureus (MTCC 96) and Bacillus cereus (MTCC 430) and two gram-negative bacteria: Pseudomonas aeruginosa (MTCC 3163) and Escherichia coli (MTCC 77) were obtained from Institute of Microbial Technology, Chandigarh, India and used for the antibacterial activity. The overnight cultures of test bacteria were subsequently adjusted to 0.5 McFarland standards. The 100µl of test bacteria were inoculated over the MHA plates using a sterile cotton swab. All the isolated endophytic fungal isolates were cultivated on PDA for 7 days at 25±2°C. The mycelial discs (6mm in diameter) were cut from the growing margins of endophytic fungi using sterile cork borer and transferred to MHA plates seeded with the test bacteria. The plates were incubated at 37°C for 24 h. Each experiment was carried out in triplicate and antibacterial activity was assayed by measurement of zone of inhibition (ZOI) in millimeters.
Statistical analysis
All the experiments were carried out in triplicate. Data were recorded as the average of three independent readings for each of the experiments. The results were subjected to standard error.
RESULTS
Isolation and identification of endophytic fungi
In present study, fungal endophytes from C. longa were isolated followed by the application of different sterilization treatments. A total of 38 endophytic fungi of different genus and species were isolated from the rhizome, roots, stem and leaves of healthy plant among which 16 (CLZ1-CLZ16, where CLZ indicates Curcuma longa Rhizome) were recovered from the rhizome, 5 (CLR1-CLR5, where CLR indicates Curcuma longa Roots) were isolated from roots, 8 (CLS1-CLS8, where CLS indicates Curcuma longa Stem) from the stem, and 9 (CLL1-CLL9, where CLL indicates Curcuma longa Leaf) were isolated from leaves after surface sterilization treatment (Table 2). Fungal endophytes act as chemical synthesizers within the plant system and have been studied from several unexplored environments till date. The recovery of fungal endophytes largely depends upon the time and concentration of sterilant used. These results support the findings that sequential treatment with 70% ethanol for 3min, 0.5% NaOCl (sodium hypochlorite) for 3 min and 70% ethanol for 30s can be helpful in the recovery of bacterial endophytes from healthy and fresh rhizomes of C. longa [1]. In the present study, lower concentration of sterilizing agents resulted in a higher contamination percentage, whereas higher concentration, on the other hand, led to lower incidence of contamination as well as a lower rate of survival (Figure 2). C. longa explants treated with 70% ethanol for one minute, 2% sodium hypochlorite for 3 min., and 70% ethanol for 30 seconds showed higher percentage of survival. All the isolated endophytic fungi were characterized based on their morphological characteristics and their reproductive structures. The isolates were identifying as Aspergillus sp., Penicillium sp., Fusarium sp., and Cladosporium sp. Reproductive structures were absent in two strains and considered as mycelia sterilia while two were remain unidentified.
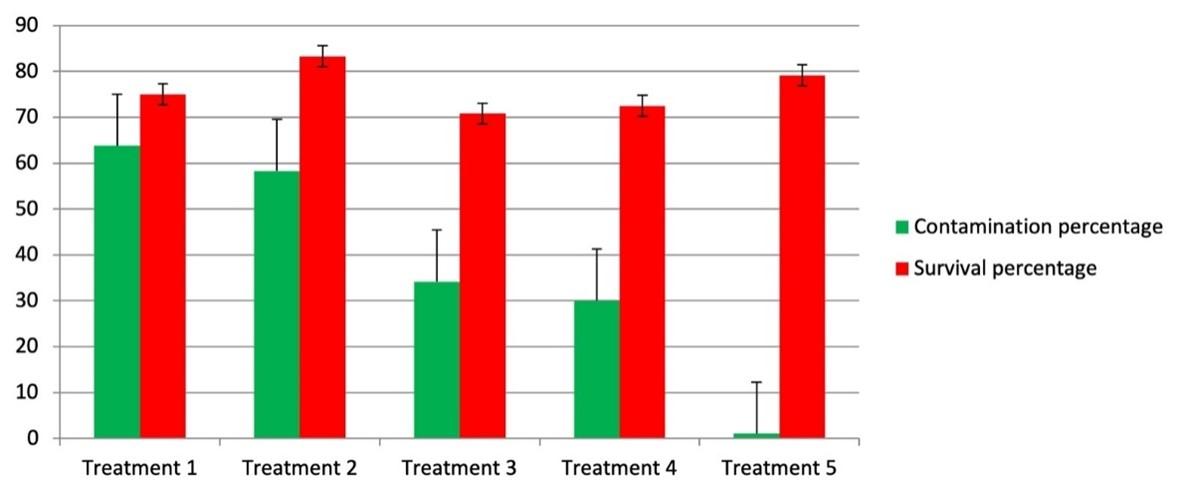
Table 2. No. of endophytic fungi isolated per part of plant.
Efficiency of surface sterilization
Four different methods namely imprinting of sterilized plant tissue, culturing the final aliquots onto the specific media, and dipping the surface sterilized plant tissue into the nutrient broth indicates the efficiency of the surface sterilization method. The absence of spores and hyphae when observed under microscope also suggest that the sterility check was complete. There was no growth observed after 21 days of incubation in sterility check (Figure 3). No turbidity in nutrient broth was observed (Table 3).
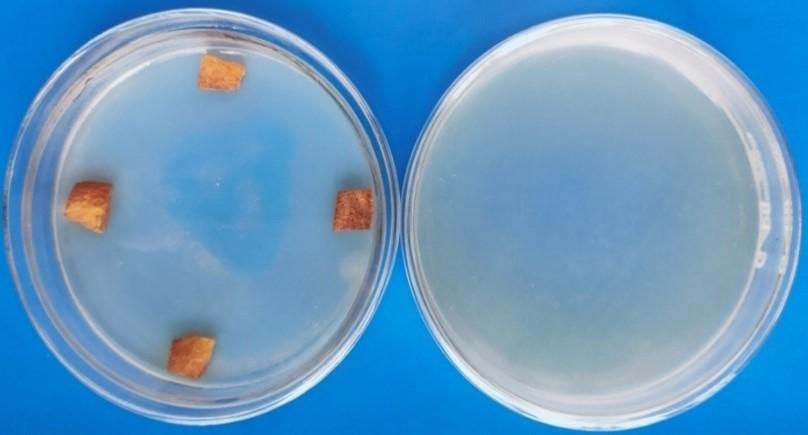
Table 3. Efficiency of surface sterilization after 21 days treatment period of Curcuma longa L.
Effect of culture medium on endophytic growth
The diversity of cultivable endophytic fungi largely depends upon the type of media used to recover fungal endophytes after sterilization treatment [14]. Three different media were used to isolate endophytic fungi from different parts of C. longa. The maximum number of endophytes were recovered from the rhizome, followed by leaf, stem, and with the least number of isolates from the roots (Figure 4, 5).
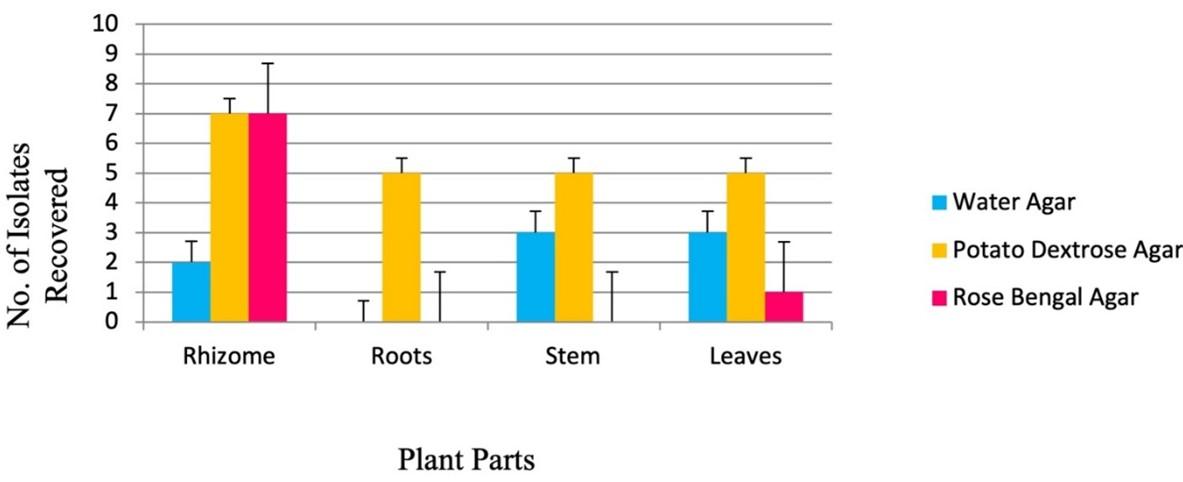
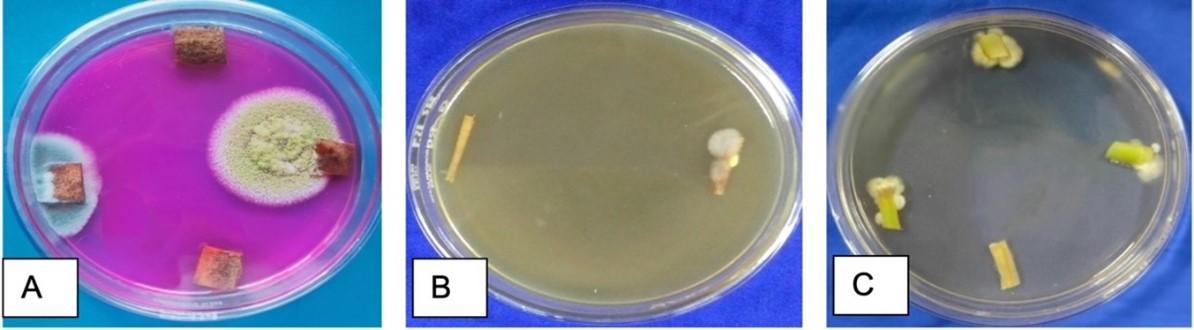
Screening of antibacterial activity by agar disc diffusion method
A total of 38 endophytic strains were isolated from different parts of C. longa and subjected for preliminary antimicrobial screening on solid medium. Of the 38 isolates examined, only 11 (28.9%) isolates displayed inhibitory activity against one or more test pathogens while other isolated endophytic fungi showed no antibacterial activity at all (Table 4). Aspergillus sp. 1, Aspergillus sp. 2, sterlia mycelia 1 and unidentified 2 sp. showed broad spectrum activity by inhibiting all the test pathogens, i.e., E. coli, S. aureus, P. aeruginosa and B. cereus. Cladosporium sp. showed inhibitory activity against E. coli, while Fusarium sp. was able to inhibit only gram-positive bacteria. Other strains were also found to be active. The obtained results are shown in (Figure 6).
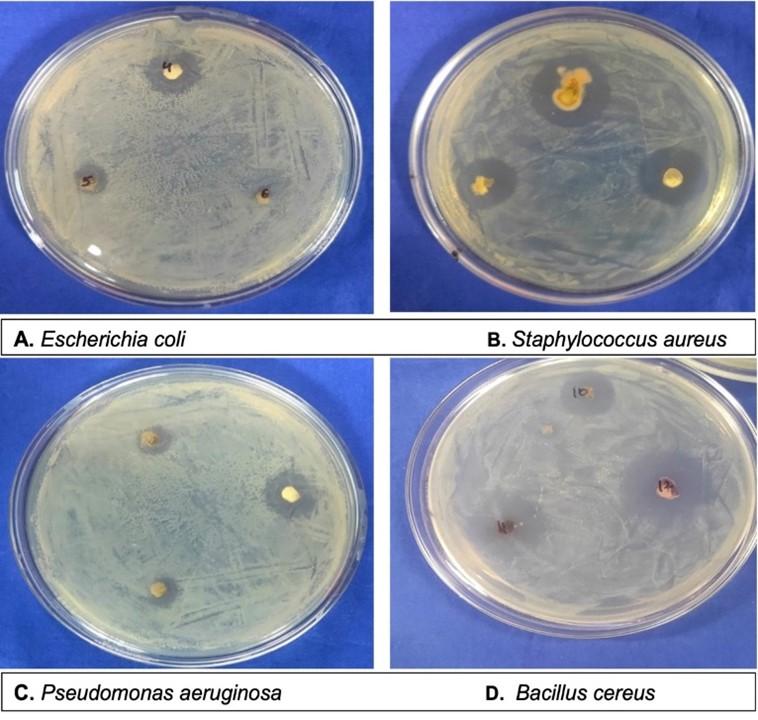
Table 4. Antibacterial activity by endophytic fungi against test pathogens.
DISCUSSION
Curcuma longa is an Indian traditional medicinal plant with antimicrobial, antioxidant, antidiabetic, antihypertensive, anti-obesity, and anti-inflammatory activities [24]. Endophytic isolation from such medicinal plants is of great importance as they mimic the host biochemical pathway for the synthesis of bioactive metabolites and provide protection to plants against plant pathogens [23]. In the present work, different sterilization treatments and media were analyzed to obtain the diversity of cultivable endophytic fungi. A total of 38 endophytic fungi were isolated from different parts of C. longa and subjected to their antimicrobial activity by agar disc diffusion method. Five different treatments were assessed for surface sterilization (T1-T5). Surface sterilization with 70% ethanol (T1) does not show any significant results in eliminating the contaminating microorganisms and was sporadically inactive. Our findings supported the study of [12] where 70% ethanol alone was not effective in the isolation of endophytic actinomycetes from medicinal plants. The application of ethanol and sodium hypochlorite in combination are the most used method to remove epiphytic microorganisms. In addition, pre-immersion of seeds from Citrus limon in sterile distilled water for 5 minutes and then surface sterilization with 70% ethanol and 2.5% sodium hypochlorite is preferable for the recovery of bacterial and fungal endophytes [25]. Furthermore, different plant tissues require different treatment as higher concentration of sterilants may damage the host tissue and have impact on endophytic fungal communities also [26]. Hence the efficiency of chemical sterilants were evaluated for removal of epiphytes from rhizome, roots, stem and leaves of C. longa found in the foothills of the Himalayas, the Tarai region in Uttarakhand, which is a typical low-lying territory flooded with water, valley, basin, marshy ground, marsh, swamp, and meadow.
In our study, surface sterilization of the stem was done using 2% sodium hypochlorite for 2 minutes followed by 70% ethanol for 30 seconds. According to a study on Phaseolus vulgaris, Pisum sativum, and Hordeum vulgare, 70% ethanol, 2% sodium hypochlorite and 0.1% mercuric chloride was found effective in the recovery of bacterial endophytes [27]. However, in our study, 70% ethanol for 1 minute for leaves, 2 % sodium hypochlorite for 3 minutes for leaves, stems, and roots, and 3 minutes for rhizome followed by 70 % ethanol for 30 seconds is effective for isolating endophytic fungi from rhizome with increased survival and decreased contamination rate at the same time. The L-asparaginase producing endophytic fungi has been recovered by sterilizing the plant material with 5% sodium hypochlorite for 3 to 8 min followed by 75% ethanol for 1 min [28]. Subsequently, 0.5% of sodium hypochlorite was found effective in isolation endophytic fungi from Solanum lycopersium [29] but in our study, high concentration of sodium hypochlorite (2%) was found efficacious in eliminating epiphytic mycoflora. Also, the amalgamation of 96% ethanol, with 1% sodium hypochlorite and 3% mercuric chloride was found effective in obtaining the culturable endophytes (bacteria, fungi and actinomycetes) from medicinal plants in Vietnam [30]. Our results indicated that the individual treatments significantly resulted in the higher contamination percentage than the combination of all sterilizing agents. Also, increased ethanol concentration, sodium hypochlorite concentrations and exposure times are not suitable for isolation procedures; it can damage the host by penetrating inside the tissues and hamper the growth of cultivable endophytic fungi.
Medicinal plants and their phytoconstituents have gigantic therapeutic applications against a variety of ailments [31]. Bioactive compounds from medicinal plants are a copious source of antimicrobial agents with no toxicity on cells. Hence, endophytic fungi from such medicinal plants are a pivotal source of novel secondary metabolites. The major compounds for antimicrobial activity from endophytic fungi include fatty acids, fatty acid esters, tertahydrofurans and sterols [32]. Our results revealed that C. longa from Haridwar region could be exploited as a source of endophytic fungi with potential antimicrobial producers.
Although all parts of C. longa have been used in traditional formulations to treat a variety of ailments, we isolated endophytic fungi from healthy rhizome, roots, stem and leaves of the plant. In the present work, all the isolated endophytic fungi from C. longa were evaluated for their antimicrobial activity. Whereas, in an earlier study, 11 fungal endophytes recovered from turmeric plants were subjected to antimicrobial activity belonging to the phylum Ascomycota and Fusarium [15]. However, 31 fungal endophytes isolated from healthy turmeric rhizomes and virulent pathogenic strains (P. aphanidermatum PyDOB-4 and R. solani RhsDOB-3) from diseased rhizome and leaf tissue [17]. It is noteworthy that 16 endophytic fungal species were obtained from rhizome and only 5 species isolated from roots. Furthermore, 9 and 8 species were cultured from leaves and stem, respectively. The results also depicted that different plant tissues harbored different endophytic species which may be due to the availability of nutrients.
In recent years, endophytic fungi have emerged themselves as a powerful source for producing novel compounds with tremendous biological activities. In our research, out of 38 endophytic fungal species, only 11 (28.9%) were found to have broad-spectrum antibacterial activity. The antimicrobial nature of endophytic fungus is due to the presence of some inhibitory compounds and hence further study is required to perform for the production and isolation of bioactive compounds. Similar results were observed in a study where 23 out of 113 endophytic fungal strains displayed antimicrobial activity isolated from the leaf and stem of Zanthoxylum simulans . Studies on antimicrobial activity by endophytic fungi also revealed certain compounds responsible for antimicrobials including, isobenzafuranones, and isocoumarins, alkaloids, phenolics, terpenes, and saponins . Also, the production of antimicrobial compounds largely varies with the incubation temperature and type of the medium used.
Furthermore, our results also indicated that endophytic fungi from C. longa have pharmaceutical potential to produce new antimicrobial compounds and the antimicrobial nature of the plant may be due to the repercussions of production of bioactive metabolites by endophytic fungi.
CONCLUSION
In the present study, optimization of surface sterilization treatment enhances the diverse occurrence of endophytic mycoflora from traditional medicinal plant (Figure 7). Furthermore, the observations of this study indicate that endophytic fungi from C. longa are potential source of antimicrobial compounds. Further, antioxidant, antidiabetic, and immunomodulatory activities will need to be included in future research.
ACKNOWLEDGEMENT
Authors are thankful to the Department of Microbiology, Kanya Gurukula Campus, Gurukula Kangari (Deemed to be University), Haridwar for providing all the essential facilities for conducting the present study.
AUTHORS CONTRIBUTIONS
HD; designed and performed the experiments; collected reagents, material analysis tools and recorded all data, write manuscripts and improved accordingly. VV; reviewed the data and the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Kumar A, Singh AK, Kaushik MS, Mishra SK, Raj P, Singh PK, Pandey KD. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: a review. 3 Biotech. 2017; 7(6):1-8.
- [2]Hay, E., Lucariello, A., Contieri, M., Esposito, T., De Luca, A., Guerra, G., & Perna, A. Therapeutic effects of turmeric in several diseases: An overview. Chem Biol Interact. 2019; 310, 108729.
- [3]Hamidpour R, Hamidpour S, Hamidpour M, Sohraby M, Hamidpour R. Turmeric (Curcuma longa): from a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-cancer, and anti-diabetes. Int J Pharmacol Phytochem Ethnomed. 2015; 1(1): 37-45.
- [4]Kim H, Ban I, Choi Y, Yu S, Youn SJ, Baik MY, Lee H, Kim W. Puffing of turmeric (Curcuma longa L.) enhances its anti-inflammatory effects by upregulating macrophage oxidative phosphorylation. Antioxidants. 2020; 9(10):931.
- [5]Yadav RP, Tarun G. Versatility of turmeric: A review the golden spice of life. J. Pharmacogn. Phytochem. 2017; 6(1):41-6.
- [6]Azeez TB, Lunghar J. Antiinflammatory effects of turmeric (Curcuma longa) and ginger (Zingiber officinale). Inflammation and Natural Products. 2021; 1:127-146.
- [7]Passari AK, Mishra VK, Saikia R, Gupta VK, Singh BP. Isolation, abundance and phylogenetic affiliation of endophytic actinomycetes associated with medicinal plants and screening for their in vitro antimicrobial biosynthetic potential. Front. Microbiol. 2015; 7, 6:273.
- [8]Matsumoto A, Takahashi Y. Endophytic actinomycetes: promising source of novel bioactive compounds. J. Antibiot. 2017; 70(5):514-519.
- [9]Poveda J, Abril-Urias P, Escobar C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020; 25;11:992.
- [10]Anjum N, Chandra R. Endophytic bacteria: optimizaton of isolation procedure from various medicinal plants and their preliminary characterization. Asian J. Pharm. Clin. Res. 2015; 8(4):233-8.
- [11]Waheeda K, Shyam KV. Formulation of novel surface sterilization method and culture media for the isolation of endophytic actinomycetes from medicinal plants and its antibacterial activity. J. Plant. Pathol. Microbiol. 2017; 1:8(399):2.
- [12]Sahu PK, Tilgam J, Mishra S, Hamid S, Gupta A, Verma SK, & Kharwar RN. Surface sterilization for isolation of endophytes: Ensuring what (not) to grow. Journal of Basic Microbiology. 2022.
- [13]Ramalashmi K, Prasanna VK, Magesh K, Sanjana R, Siril JS, Ravibalan K. A potential surface sterilization technique and culture media for the isolation of endophytic bacteria from Acalypha indica and its antibacterial activity. J Med Plant Stud. 2018; 6:181-4.
- [14]Septiana E, Sukarno N, Simanjuntak P. Endophytic fungi associated with turmeric (Curcuma longa L.) can inhibit histamine-forming bacteria in fish. Hayati. J. Biosci. 2017; 1:24(1):46-52.
- [15]Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003; 67(4):491-502.
- [16]Vinayarani G, Prakash HS. Fungal endophytes of turmeric (Curcuma longa L.) and their biocontrol potential against pathogens Pythium aphanidermatum and Rhizoctonia solani. World J. Microbiol. Biotechnol. 2018; 34(3):1-7.
- [17]Petrini O. Taxonomy of endophytic fungi of aerial plant tissues. Microbiology of the Phyllosphere. 1986.
- [18]Schulz B, Wanke U, Draeger S, Aust HJ. Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol. Res. 1993; 1:97(12):1447-50.
- [19]Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. Illustrated genera of imperfect fungi.. 1972(3rd ed).
- [20]Ellis MB. Demataceous Hyphomycetes; Commonwealth Mycological Institute: Kew, UK, 1976.
- [21]Forbes BA, Sahm DF, Weissfeld AS. 2016. Study Guide for Bailey and Scott’s Diagnostic Microbiology-E-Book. Elsevier Health Science.
- [22]Faddetta T, Abbate L, Alibrandi P, Arancio W, Siino D, Strati F, De Filippo C, Fatta Del Bosco S, Carimi F, Puglia AM, Cardinale M. The endophytic microbiota of Citrus limon is transmitted from seed to shoot highlighting differences of bacterial and fungal community structures. Sci Rep. 2021; 29;11(1):1-2.
- [23]Hatamzadeh S, Rahnama K, Nasrollahnejad S, Fotouhifar KB, Hemmati K, White JF, Taliei F. Isolation and identification of L-asparaginase-producing endophytic fungi from the Asteraceae family plant species of Iran. PeerJ. 2020; 14;8:e8309.
- [24]Mohammad Golam Dastogeer K, Oshita Y, Yasuda M, Kanasugi M, Matsuura E, Xu Q, Okazaki S. Host specificity of endophytic fungi from stem tissue of nature farming tomato (Solanum lycopersicum Mill.) in Japan. Agronomy. 2020; 10(7):1019.
- [25]ran HM, Nguyen DT, Mai NT, Do HT, Nguyen TK, Nguyen TK, Muller M, Nguyen HQ. Notes on Culturable Endophytic Microorganisms Isolated from 14 Medicinal Plants in Vietnam: A Diversity Analysis to Predict the Host-Microbe Correlations. Curr Microbiol. 2022; 79(5):1-0.
- [26]Archana H, Bose VG. Evaluation of phytoconstituents from selected medicinal plants and its synergistic antimicrobial activity. Chemosphere. 2022; 1;287:132276.
- [27]Sharaf MH, Abdelaziz AM, Kalaba MH, Radwan AA, Hashem AH. Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from ocimum basilicum. Appl Biochem Biotechnol. 2022; 194(3):1271-89.
- [28]An C, Ma S, Shi X, Xue W, Liu C, Ding H. Isolation, diversity, and antimicrobial activity of fungal endophytes from Rohdea chinensis (Baker) N. Tanaka (synonym Tupistra chinensis Baker) of Qinling Mountains, China. PeerJ. 2020; 17;8:e9342.
- [29]Kuo J, Chang CF, Chi WC. Isolation of endophytic fungi with antimicrobial activity from medicinal plant Zanthoxylum simulans Hance. Folia Microbiologica. 2021; 66(3):385-97.
- [30]Chen Q, Yu JJ, He J, Feng T, Liu JK. Isobenzofuranones and isocoumarins from kiwi endophytic fungus Paraphaeosphaeria sporulosa and their antibacterial activity against Pseudomonas syringae pv. actinidiae. Phytochemistry. 2022; 1;195:113050.
- [31]Du W, Yao Z, Li J, Sun C, Xia J, Wang B, Shi D, Ren L. Diversity and antimicrobial activity of endophytic fungi isolated from Securinega suffruticosa in the Yellow River Delta. PloS one. 2020; 10;15(3):e0229589.
- [32]Castro P, Parada R, Corrial C, Mendoza L, Cotoras M. Endophytic Fungi Isolated from Baccharis linearis and Echinopsis chiloensis with Antifungal Activity against Botrytis cinerea. J Fungi. 2022; 18;8(2):197.