Study on relationship between genetic abnormalities and clinicopathological features in K hospital’s patients with colorectal cancer
Abstract
The MAPK-ERK, as well as PI3K-AKT signaling transduction pathway, represents a pivotal function in tumorigenesis. Genetic alterations of potential tumor-driven genes, for instance, KRAS, BRAF, NRAS, and PIK3CA can result in uncontrolled cell proliferation and progression. The main aims of the study were not only to identify the prevalence of KRAS, BRAF, NRAS, PIK3CA molecular modifications but also to evaluate the relationship between gene changes and clinical and/or pathological characteristics of 251 Vietnamese colorectal cancer. Genetic abnormalities on KRAS, BRAF, NRAS, and PIK3CA were detected through the utility of Realtime PCR, Pyrosequencing, and Direct sequencing methods, respectively. The frequency of KRAS, BRAF, NRAS, and PIK3CA mutations were 34.3%, 6.4%, 7.2%, and 17.5%, in turn. KRAS mutation was mutually exclusive against that of NRAS and BRAF mutations in CRC. BRAF, as well as RAS/RAF mutations, were more usual in older age. A significant association between PIK3CA mutations and age together with differentiation of CRC was determined. In addition, PIK3CA mutation tended to coexist with KRAS but not with NRAS and BRAF mutation. Our results indicate the information of molecular markers that contribute to self-sufficient oncogenic mechanisms in the carcinogenesis of CRC.
INTRODUCTION
Worldwide, colorectal cancer (CRC) is one of the majority types of cancer, which is the third most widely examined and the fourth malignant neoplastic disease-related mortality. The percentage of Caucasians CRC has been given a picture of being more superior to the Asian Ethnic. For the time being, the occurrence of cancer from parts of the large intestine was considerably accelerated in Asian countries including China, the Republic of Korea, and Vietnam, and there is a speedily rising tendency in the future, which may potentially be related to risk elements such as nutritional factors, diet modification, physical inactivity, the habit of smoking and extravagant alcohol dependence and environmental contamination [1, 2].
Activating mutations in the RAS-RAF-MAPK pathway including KRAS, BRAF, and NRAS abnormalities have been demonstrated to be major prognostic factors about resistance in the expectation of anti- Epidermal Growth Factor Receptor (anti-EGFR) medications. Patients with wild-type KRAS, NRAS, and BRAF display clinical sensitivity to this targeted therapy [3]. Since it’s important to determine RAS/RAF mutation before using cetuximab and panitumumab. This allows us to precisely predict the efficacy of anti-EGFR monoclonal antibodies (mAb) as well as understand the molecular characteristics of CRC [3].
In addition, Phosphoinositide-3-kinase (PI3K) is the family of lipid kinases in the PI3K/AKT/mTOR transduction route, that assumes a variety of cellular functions and is often dysregulated in solid tumors. Abundant studies have been evidence of activated tumor-derived PIK3CA mutations were observed in many malignancies including CRC [4, 5]. PIK3CA mutation is present in 10-20% of colorectal cancer, in which approximately 80% of variant regions on the subject of the helical along with kinase domains of exon 9 and 20, correspondingly [5]. The PIK3CA mutation is closely associated with KRAS mutations and epigenetic modifications, in particular coincidental hypermethylation of numerous CpG-rich promoters of several genes (the CpG island methylator phenotype, or CIMP) [6]. Monoclonal antibody drugs targeting EGFR such as cetuximab and panitumumab are major target therapy in malignant colorectal cancer, however, PIK3CA pathogenic variant carriers could potentially belong less susceptible toward these target drugs [7]. This suggests that genetic abnormalities of RAS/RAF and PI3K pathway should be evaluated to guide the anti-EGFR treatment. Furthermore, identifying interactions between genetic changes in KRAS, BRAF, NRAS, and PIK3CA oncogenes may help to understand the detailed carcinogenesis mechanism of colorectal tumors, in addition to explaining differences in healing response among individual patients. RAS, RAF, and PIK3CA abnormalities induce to activate of the MAPK and PI3K signaling transduction paths, resulting in the interior of consolidative or conglomerative impact on the edge of being alive of CRC sufferers [8, 9]. Although new insights into the mechanisms have emerged from recent studies, information about molecular changes in Vietnamese CRC patients remains unclear. Hence, this research was designed to meet the needs of frequency in tandem with the dispensation of genetic variations in KRAS, NRAS, BRAF along PIK3CA, on top of that correlation of each with the clinicopathological parameters of the Vietnamese CRC population.
MATERIALS AND METHODS
Obtaining tissue specimens
During the time between Nov 2019 and Oct 2021, we gathered 251 formalin-fixed paraffin-embedded (FFPE) clinical blocks according to the criteria each sample was pathological diagnosed based on the American Joint Committee on Cancer (AJCC) and operated surgical intervention on the edge of National Cancer Hospital K in Vietnam. The patient’s tumor samples used in the study were not only obtained informed consent but also licensed all through the ordinances of the Vietnamese morality commission (Circular No.04/2008/TT-BYT). Sections (5μm thick) were cut from paraffin-embedded tumor tissue blocks and stained with Hematoxylin & Eosin using the Thermo Fisher Scientific system for histopathological examination, following the manufacturer’s protocol.
DNA isolation from CRC tissue
QIAamp DNA FFPE Tissue Kit (Qiagen) was utilized for genomic DNA extraction from formalin-fixed paraffin-embedded tissues. The quality of DNA specimens was evaluated utilizing polymerase chain reaction (PCR) which amplified a single-copy gene, β-globin. Besides, the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific) allows resolving the total DNA amount for this study.
Investigation of KRAS, BRAF, NRAS, and PIK3CA genetic changes from CRC tissue
Cobas® KRAS Mutation Test, Cobas® 4800 BRAF V600 Mutation Test (Roche) together with therascreen NRAS Pyro Kit (Qiagen) were used to identify mutations of KRAS exon 2-3; BRAF V600 on exon 15 and NRAS exon 2-3, respectively. PIK3CA transformations in the interior of the exon 9 in tandem with 20 were discovered through the utility of 3130 Genetic Analyzer (Applied Biosystems). All procedures were exactly performed as mentioned by the manufacturers’ instructions. Primer sequences were detailed inward of Table 1.
Table 1. Primer sequences used for the study.
Statistical analysis
The frequency of KRAS, BRAF, NRAS, and PIK3CA alterations accompanying the correlation between genetic abnormalities, and clinicopathological characteristics of colorectal cancer was evaluated by SPSS software version 20.0. In the present study, the association of variables is measured through the utility of the Fisher’s exact test or else χ2 test. The probability meaning in the expectation of the entirety of experiments was established at p < 0.05.
RESULTS
Clinicopathological parameters of patients with colorectal cancer
Clinicopathological features of 251 CRC patients in this study were showed in Table 2. Among 251 patients, the intermediate-age getting on for diagnosis was 59.3 years (ranging from 26 to 90 years). On the other hand, the proportion in respect to male to female patients was 1.28. Two hundred fifty-one patients with colorectal cancer including 136 (54.2%) and 115 (45.8%) were collected from the colon and rectum, respectively. Based on histological category, there were 187 (74.5%) adenocarcinoma (A), 54 (21.5%) mucinous adenocarcinoma (MA), 7 (2.8%) squamous cell carcinoma (SCC), and 3 (1.2%) signet ring cell carcinoma (SRCC) (Figure 1). As for tumor differentiation, 12 (4.8%) were well-differentiated, 160 (63.7%) moderately differentiated, and 15 (6.0%) poorly differentiated (excluding 54 mucinous adenocarcinomas, 7 squamous cell carcinomas, and 3 signet ring cell carcinoma). In our study, a predominant part of tumors (71.7%) was smaller than 5 cm in measurement, with a balanced lymph node metastasis status ratio. Pathologic stages showed 4 (1.6%) cases within stage I, 112 (44.6%) cases enclosed by stage II, 115 (45.8%) sufferers in stage III, in tandem with 20 (8.0%) patients in stage IV (Table 2).
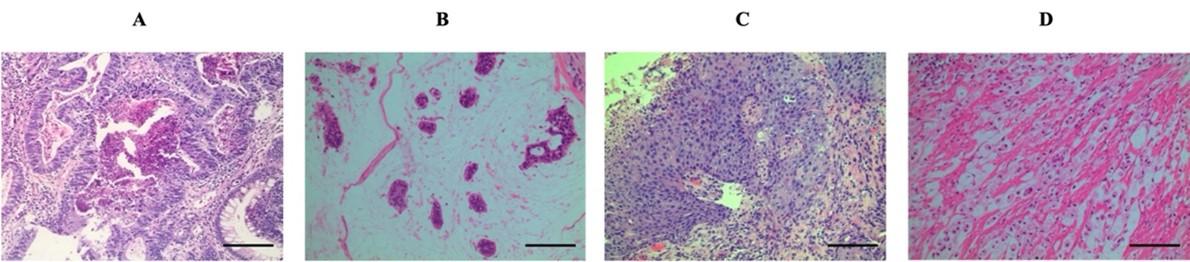
Table 2. Clinical and pathological parameters in the patients owing to CRC.
The rate together with the distribution of KRAS, NRAS, BRAF, and also PIK3CA mutations in addition to their interrelations with clinicopathologic characteristics in patients with CRC
Table 3 detailed genetic abnormalities in 251 CRC tissue blocks. Our results showed that 86 cases had KRAS mutation, reaching 34.4%, which include 77 patients who harbored mutation situated at codon 12/13 belonging to the exon 2 combined with 9 sufferers found in codon 61 coming out of the exon 3. 17 out of 18 (6.8%) NRAS alterations were distributed in adenocarcinoma. Of 18 NRAS mutations, a greater part of changes was found at codon 12/13 from the exon 2 with regards to 14 patients, achieving 77.8%. Only 4 patients carried an NRAS missense mutation at codon 61 from exon 3. There was no meaningful interaction amongst KRAS variations, NRAS mutation along with clinical and pathological features (agedness, sexual characteristics, tumor position, histological subtypes, differentiation, lymph node metastasis, tumor dimensions, and stage). BRAF mutation occurred in 6.4% (16/251) of cases in codon 600 of the exon 15. Compared with RAF wild-type tumors, BRAF mutant tumors were statistically associated with the younger group (p=0.023) (Table 3).
The mutation of either RAS (KRAS and NRAS) or BRAF was detected in 47.8% (120/251) of the cases examined. A critical correlation inward of RAS/RAF modifications with patients’ age was observed within the bounds of our present study (p= 0.032). Regarding pathological parameters, RAS/RAF alterations tended also to be lightly correlated with histological subtypes (p=0.058), differentiation level (p=0.060), and lymph node malignancy status (p=0.059). Whereas RAS/RAF genetic changes in tandem with other clinicopathological features including patients’ gender, tumor location, tumor size, and stages did not show any association in CRC tumors (p>0.05) (Table 3).
In addition, all data according to the rate in parallel with distribution concerning PIK3CA genetic changes were exhibited within the interior of this study. PIK3CA modification was identified in 44 samples (17.5%), of which 75.0% (33/44) and 25.0% (11/44) were occurred in exon 9 (including 7 E542 and 26 E545) and exon 20 (including 3 H1046, and 8 H1047), respectively (data not shown). The association between PIK3CA variant standing and clinical at the same time as pathological characteristics was not found in the Vietnamese patients with CRC. In contrast, a genetic abnormality in the PIK3CA gene had a higher incidence among males and moderately differentiated tumors (p<0.05).
Table 4 illustrated the interrelationship between somatic alterations of KRAS, BRAF, NRAS, and PIK3CA gene. Our results confirmed that KRAS mutation exhibited a mutually exclusive with NRAS and BRAF mutation pattern in CRC and was a strong association with PIK3CA mutation (p< 0.05). Meanwhile, no statistical correlation was found between BRAF and/or NRAS and PIK3CA mutations (p> 0.05).
Table 3. KRAS, NRAS, BRAF and PIK3CA somatic variations, as well as interrelationships together with clinical and pathological features.
Table 4. Correlation with regards to KRAS, NRAS, BRAF and PIK3CA abnormalities in CRC.
DISCUSSION
The EGFR signaling transduction path is involved in many important functions inside the range of cells, which dysregulate to lead to uncontrolled growth, appearing in solid cancers, including CRC [10]. Based on genetic alterations of this signaling pathway, cetuximab, panitumumab, nimotuzumab, and necitumumab is a group of targeting as concerns EGFR using a monoclonal antibody that has significantly improved the treatment, especially for patients with metastatic CRC [11]. However, De Roock W et al. (2010) confirmed that genetic changes belonging to KRAS, BRAF, NRAS, and PIK3CA genes were related further to a lower response rate after making utilization done by anti-EGFR monoclonal antibodies [12].
The frequency in regard to KRAS, BRAF, NRAS, and PIK3CA modifications was found in 86 of having 251 (34.3%), 16 of 251 (6.4%), 18 of 251 (7.2%), and 44 of 251 (17.5%) patients examined, respectively. Interestingly, there were 135 (53.8%) cases of patients who carried an oncogenic mutation in at the minimum one gene, including KRAS, BRAF, NRAS, and/or PIK3CA. Our results indicated that out of 165 KRAS wild-type CRC patients, 49 (29.7%) harbor NRAS, BRAF, or PIK3CA mutations. The reported KRAS mutation rate in patients with CRC varies widely between different populations worldwide, ranging from 13% to 66% [13–16]. In Vietnamese research, KRAS missense mutations at codons 12, 13, and 61 were detected in 34.3% of patients about CRC. Inside the range of our study, we discovered 34.3% of suffers harbored KRAS mutations, which was concordant according to reported data deriving out of Asian countries (i.e., China, Japan, and India) (20–66%), and lower than the one revealed surrounded by TCGA data (42%) [17].
Rat sarcoma virus (RAS) family members take the part of a key function in cell development. Any activating mutation at the hand of the RAS family, including KRAS, NRAS, and HRAS is an appropriate target for anticancer therapy [18]. Before the present time, there is a minority of studies on the subject of the prevalence of NRAS genetic modifications, ranging from 2.0 % to 10.0 % [14,16,19]. The frequency of NRAS mutations was 7.2% of the Vietnamese CRC patients. Similar to KRAS mutations, there was no meaningful relationship between NRAS mutations and clinical parameters were indicated in CRC tissue blocks. The extensive variability in frequency, as well as distribution of KRAS and also the NRAS mutation between studies, may perhaps be due to ethnicity, geographic factor, sample size, and mutation analysis techniques.
BRAF gene composes of 18 exons, which performs the function of a downstream signal transduction component of triggering of the mitogen-activated protein kinase (MAPK) signal transduction. BRAF V600E (exon 15) is the most common activating mutation, interprets as 90% of the aggregate activating BRAF pathogenic variations [20]. All over the world, the described rate appertaining to BRAF alterations inward of dissimilar inhabitants fluctuates broadly, from 1.1% to 25% [13–16]. Within the confines of this study, the V600E BRAF variation was discovered in 16 patients, employing a percentage of 6.4% (16/251), which is more lightly outstanding than different Asian publications (1.1% to 5.8%). For the BRAF gene, of extraordinary consideration is the fact that the incidence of V600E mutation gave variety to in terms of age, as far as an outstandingly higher proportion in older convalescents (5.2%) compared to that in younger patients (1.2%) (p=0.023), it was similar to the previous report, showed that BRAF V600E mutation escalated from 10% in the interior of unselectable cases to 37% enclosed by females elder than the 70s [21].
Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) belongs to the PI3K family that is frequently mutated in solid tumors. In the present research, the rate of PIK3CA genetic changes was found in 44 patients, reaching 17.5% (44/251), consistent with the prevalence from 2% to 18% of metastatic CRCs [13,14,15]. Our study showed a significant association between PIK3CA mutation and patients’ gender and differentiation (p<0.05). Ziv E et al. (2017) indicated that PIK3CA or AKT mutation carriers laid hold of poorer disease progression (55%) than wild-type groups (92%) after radiation, at 1-year post-embolization [22]. This finding suggests that activating mutations belonging to the PI3K signal transduction, especially PIK3CA genetic abnormalities, may potentially affect radiotherapy for CRC patients.
Our present study confirmed that KRAS mutation excludes NRAS and BRAF missense variations in CRC (p< 0.05), suggesting genetic alterations are involved in different oncogenic pathways for colorectal cancer tumorigenesis. This result could potentially be explained by the incompatibility between the mutations, just 1 mutation within the interior of the MAPK signaling pathway is enough to put a stop to the cell cycle [23]. Some genetic alterations may coexist, others are exclusive, such as the coexistence of KRAS mutations and APC inactivation leading to CRC progression [24]. Meanwhile, BRAF and APC pathogenic modifications are rarely found together in CRC. In the earliest precursor of CRC and adenomas, a considerable correlation out of BRAF alteration along with the serrated histological characteristic was detected [25]. In addition, we inaugurated a strong interrelationship between PIK3CA and KRAS mutations; PIK3CA to go with RAS/RAF mutations, similar to previous reports. For example, Li HT et al. (2011) indicated that KRAS and PIK3CA somatic co-variations are more popular surrounded by patients abreast of stage IV CRC than the early stages. This may be due to the complementary impact of mutations leading to activating the PI3K-AKT signaling pathway, resulting in metastasis [26]. Once KRAS/PIK3CA mutations are coexistence in the early stage, the patient has a poor prognosis such as developing distant metastasis and worse outcome [27]. Patients carrying mutations that activate the PI3K signaling pathway are commonly less susceptible to targeted therapy using anti-EGFR monoclonal antibodies. Thus, in addition to RAS/RAF mutations, the mutation status of components involving the PI3K signaling pathway is considered a biomarker for negative prognosis when it comes to anti-EGFR monoclonal antibodies therapy to approach progressive colorectal cancer.
In conclusion, our present study demonstrated the specific associations of alterations with KRAS, NRAS, BRAF, PIK3CA gene, and CRC patients’ clinicopathologic parameters, suggesting to help individualized patient-oriented treatment for cancer patients. Our results assist in better characterizing the Vietnamese CRC population to better announce to clinicians and researchers. Future molecular detailed studies should be carried out evaluating different outcomes by oncogenic abnormalities in CRC tumors.
ACKNOWLEDGEMENTS
None.
AUTHOR CONTRIBUTIONS
L.D.V. and Q.N.N.: Conception and Design of the experiments. H.H.C.: Methodology and Data analysis, L.D.V.: Data curation and Writing – original draft, Q.N.N: Writing – review and editing, Supervision. All authors reviewed the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Rosato V, Bosetti C, Levi F, Polesel J, Zucchetto A, Negri E, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control 2013; 24:335–41. https://doi.org/10.1007/s10552-012-0119-3.
- [2]Deng Y. Rectal Cancer in Asian vs. Western Countries: Why the Variation in Incidence? Current treatment options in oncology 2017; 18: e64. https://doi.org/10.1007/s11864-017-0500-2.
- [3]Matsubara N. Epigenetic regulation and colorectal cancer. Dis Colon Rectum 2012; 55:96–104. https://doi.org/10.1097/DCR.0b013e318233a1ef.
- [4]Shaw RJ, Cantley LC. Ras, PI3K, and mTOR signaling control tumour cell growth. Nature 2006; 441:424–30. https://doi.org/10.1038/nature04869.
- [5]Ogino S, Lochhead P, Giovannucci E, Meyerhardt JA, Fuchs CS, Chan AT. Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: power and promise of molecular pathological epidemiology. Oncogene 2014; 33:2949–55. https://doi.org/10.1038/onc.2013.244.
- [6]Rosty C, Young JP, Walsh MD, Clendenning M, Sanderson K, Walters RJ, et al. PIK3CA activating mutation in colorectal carcinoma: associations with molecular features and survival. PLoS One 2013; 8: e65479. https://doi.org/10.1371/journal.pone.0065479.
- [7]Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget 2017; 8:3980–4000. https://doi.org/10.18632/oncotarget.14012.
- [8]Stintzing S, Lenz H-J. A small cog in a big wheel: PIK3CA mutations in colorectal cancer. J Natl Cancer Inst 2013; 105:1775–6. https://doi.org/10.1093/jnci/djt330.
- [9]Ganesan P, Janku F, Naing A, Hong DS, Tsimberidou AM, Falchook GS, et al. Target-based therapeutic matching in early-phase clinical trials in patients with advanced colorectal cancer and PIK3CA mutations. Mol Cancer Ther 2013; 12:2857–63. https://doi.org/10.1158/1535-7163.MCT-13-0319-T.
- [10]Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol 2007; 25:4057–65. https://doi.org/10.1200/JCO.2007.11.8984.
- [11]Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 2008; 9:962–72. https://doi.org/10.1016/S1470-2045(08)70206-7.
- [12]De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010; 11:753–62. https://doi.org/10.1016/S1470-2045(10)70130-3.
- [13]Mao C, Zhou J, Yang Z, Huang Y, Wu X, Shen H, et al. KRAS, BRAF and PIK3CA mutations and the loss of PTEN expression in Chinese patients with colorectal cancer. PLoS One 2012; 7: e36653. https://doi.org/10.1371/journal.pone.0036653.
- [14]Bando H, Yoshino T, Shinozaki E, Nishina T, Yamazaki K, Yamaguchi K, et al. Simultaneous identification of 36 mutations in KRAS codons 61 and 146, BRAF, NRAS, and PIK3CA in a single reaction by multiplex assay kit. BMC Cancer 2013; 13:405. https://doi.org/10.1186/1471-2407-13-405.
- [15]Bisht S, Ahmad F, Sawaimoon S, Bhatia S, Das BR. Molecular spectrum of KRAS, BRAF, and PIK3CA gene mutation: determination of frequency, distribution pattern in Indian colorectal carcinoma. Med Oncol 2014; 31:124. https://doi.org/10.1007/s12032-014-0124-3.
- [16]Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol 2014; 53:852–64.
- [17]Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. https://doi.org/10.1126/scisignal.2004088.
- [18]Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003; 3:11–22. https://doi.org/10.1038/nrc969.
- [19]Negru S, Papadopoulou E, Apessos A, Stanculeanu DL, Ciuleanu E, Volovat C, et al. KRAS, NRAS and BRAF mutations in Greek and Romanian patients with colorectal cancer: a cohort study. BMJ Open 2014; 4: e004652. https://doi.org/10.1136/bmjopen-2013-004652.
- [20]Wan PTC, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004; 116:855–67. https://doi.org/10.1016/s0092-8674(04)00215-6.
- [21]Tie J, Desai J. Targeting BRAF mutant metastatic colorectal cancer: clinical implications and emerging therapeutic strategies. Target Oncol 2015; 10:179–88. https://doi.org/10.1007/s11523-014-0330-0.
- [22]Ziv E, Bergen M, Yarmohammadi H, Boas FE, Petre EN, Sofocleous CT, et al. PI3K pathway mutations are associated with longer time to local progression after radioembolization of colorectal liver metastases. Oncotarget 2017; 8:23529–38. https://doi.org/10.18632/oncotarget.15278.
- [23]Sewing A, Wiseman B, Lloyd AC, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol 1997; 17:5588–97. https://doi.org/10.1128/MCB.17.9.5588.
- [24]Janssen K-P, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P, et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 2006; 131:1096–109. https://doi.org/10.1053/j.gastro.2006.08.011.
- [25]Rosenberg DW, Yang S, Pleau DC, Greenspan EJ, Stevens RG, Rajan TV, et al. Mutations in BRAF and KRAS Differentially Distinguish Serrated versus Non-Serrated Hyperplastic Aberrant Crypt Foci in Humans. Cancer Res 2007; 67:3551-4. https://doi.org/10.1158/0008-5472.CAN-07-0343.
- [26]Li H-T, Lu Y-Y, An Y-X, Wang X, Zhao Q-C. KRAS, BRAF and PIK3CA mutations in human colorectal cancer: relationship with metastatic colorectal cancer. Oncol Rep 2011; 25:1691–7. https://doi.org/10.3892/or.2011.1217.
- [27]Velho S, Moutinho C, Cirnes L, Albuquerque C, Hamelin R, Schmitt F, et al. BRAF, KRAS and PIK3CA mutations in colorectal serrated polyps and cancer: primary or secondary genetic events in colorectal carcinogenesis? BMC Cancer 2008; 8:255. https://doi.org/10.1186/1471-2407-8-255.