Antioxidant, anticholinesterase, and neurotrophic potentials of indigenous medicinal herbs of Bangladesh
Abstract
In Ayurvedic system of medicine, a variety of medicinal herbs are being prescribed for brain disorders, including mental dysfunction, indifference, and memory impairment. Neuropharmacological mechanisms of these herbs are poorly understood. A total of nineteen indigenous medicinal herbs of Bangladesh were investigated for their neuropharmacological potentials, including antioxidant, anticholinesterase, and neurotrophic activities. The antioxidant activity of plant ethanolic extracts was determined based on their DPPH free radical scavenging capacity. Acetylcholinesterase (AChE) inhibitory activity was determined by the colorimetric assay based on Ellman’s method. The neurotrophic activity of plant extracts was measured based on their capacity to promote neurite outgrowth in a primary culture of hippocampal neurons. Of the herbs, Camellia sinensis, Terminalia chebula, Cinnamomum tamala, Terminalia bellirica, Phyllanthus emblica, and Curcuma longa exhibited remarkable antioxidant activity with IC50 values of <100 µg/mL. The highest anticholinesterase activity was shown by C. longa (IC50 9.37 µg/mL) followed by C. sinensis (IC50 86.01 µg/mL) and C. tamala (IC50 86.37 µg/mL). Notably, C. tamala showed the highest neurotrophic activity (an increase in the length of primary neurites by 82% compared to control), whereas C. sinensis and C. longa showed moderate activities. The neurotrophic activity of C. tamala was reported for the first time. The present findings indicate that these indigenous herbs, particularly C. tamala, C. sinensis, and C. longa possess a remarkable neuropharmacological potential and suggest that these neuroactive herbs could be used in disease-modifying therapies for brain disorders.
INTRODUCTION
As life expectancy continues to increase, degenerative brain disorders including Alzheimer’s disease (AD) are of growing concern among elderly persons. In AD, there is a degeneration of cholinergic neurons in the basal forebrain and associated loss of cholinergic neurotransmission in the cerebral cortex and some other areas [1]. Therefore, Acetylcholinesterase (AChE) inhibitors to enhance cholinergic function in cognitive disorders to prolong the availability of acetylcholine (ACh) released into the neuronal synaptic cleft could have a therapeutic role to overcome cognitive deficits in the AD. Existing AChE inhibitors are limited by their effectiveness in only relieving symptoms and failing to halt disease progression.
Oxidative stress (OS), a pathological condition resulting from an imbalance between ROS generation and cellular antioxidant capacity is known to be implicated in AD pathobiology [2]. Antioxidants that can minimize the damaging effect of ROS may be effective in preventing or at least delaying the progression of this disease [3]. In addition, the region-specific neuronal degeneration and atrophy, with the reduction of neurotrophic factors level and excessive neuronal cell loss via neurite damage is a common pathogenic feature of AD [4, 5]. Pharmacological intervention that may induce neuritogenesis and replenish neurotrophic factor level in the brain can be beneficial for patients. Under such conditions, therapeutic agents with combined anti-AChE, antioxidant, and neurotrophic potentials could be applied as multi-target strategies against AD onset and progression.
Medicinal plants have been explored for their antioxidant, anticholinesterase and neurotrophic potentials that may offer a promising solution for neurodegenerative disorders [6, 7]. From the time immemorial, the Ayurvedic system of medicine has been using various herbs for the management of CNS ailments as well as to improve cognitive function [8]. It is widely accepted that drugs derived from plant sources are relatively well tolerated than synthetic sources. Indigenous plants that are popular in folk medicine and often used in herbal formulas for several neurological problems remain largely underexploited for their neuropharmacological properties. Aiming to discover novel and promising sources of potential anti-AD agents, the present study was designed to evaluate the antioxidant, anticholinesterase, and neurotrophic activities of indigenous medicinal plants in Bangladesh as an effective remedy for memory and other cognitive disorders.
MATERIALS AND METHODS
Chemicals
1, 1-diphenyl-2-picrylhydrazyl (DPPH), acetylthiocholine iodide (ATCI), AChE from electric eel (type VI-S lyophilized powder), bovine serum albumin (BSA), (5, 59-dithiobis [2-nitrobenzoic acid]) (DTNB), ascorbic acid and berberine were purchased from Sigma. All other reagents used were of analytical grade and obtained locally.
Plant sample collection and extract preparation
Rhizome of Curcuma longa and fresh leaves of other 18 medicinal plants were collected from the Botanical Garden of Crop Botany department, Bangladesh Agricultural University. The identification of plants and authentication of the botanical name was performed by AKMMI. The botanical names of plants have also been checked with http://www.theplantlist.org (accessed in December 2018). Voucher specimens are deposited in the corresponding author’s (MAH) laboratory. Botanical names, English names, local names, and traditional uses in folk medicines for cognitive functions of these medicinal plants are summarized in Table 1.
The plant samples were cleaned and air-dried at room temperature for two weeks. Samples were then pulverized using a grinder to a uniform powder and then sieved. The powdered sample was extracted twice with 95% ethanol (1:50 ratio). The mixture was kept on an orbital shaker at 200 RPM at RT for overnight. The supernatant was filtered and concentrated in vacuo and completely dried under a stream of nitrogen gas. The dried ethanol extract was weighed and then dissolved in EtOH or dimethyl sulfoxide (DMSO) to make an aliquot and stored in a foil-wrapped vial at -20℃ for further analysis.
Table 1. Bangladeshi medicinal herbs investigated in the current study and their traditional uses relevant to CNS function.
Determination of antioxidant property by DPPH radical scavenging assay
The antioxidant activity of plant ethanolic extracts was determined based on their DPPH free radical scavenging capacity [9]. One mL of 10 mM ethanolic stock solution of DPPH was prepared by adding 200 µL DPPH of 50 mM in 800 µL EtOH. A 40 µL ethanolic solution of DPPH (10 mM) was added to 200 µL of each extract solution of the samples with different concentrations (0.01 – 1 µg/mL). DPPH solution was freshly prepared and kept in the dark at 4℃. Ethanol (3.8 mL) was added, and the mixture was shaken vigorously. After 30 min, absorbance was measured spectrophotometrically at 517 nm. A blank sample containing the same amount of ethanol and DPPH was also prepared in each case. All determinations were performed in triplicate. The radical scavenging activities of the tested samples expressed as a percentage of inhibition were calculated according to the following equation [10].
% inhibition of DPPH activity = [(AB – AA) / AB] × 100
Where AA and AB denote the absorbance values of the test and the blank sample, respectively. The radical scavenging effect was examined and compared with natural antioxidant L-ascorbic acid (as a positive control).
Acetylcholinesterase inhibition assay
Acetylcholinesterase (AChE) activity was determined using the colorimetric microplate assay based on Ellman’s method, using acetylthiocholine iodide (ATCI) as a substrate [11]. AChE used in the assay was from an electric eel. DTNB and ACTI were dissolved in the buffer and deionized water, respectively. In the 96-well plates, 100 mL of 3 mM DTNB, 20 mL of 0.26 U/mL of AChE, and 40 ml of buffer (50 mM Tris pH 8.0), 20 mL of each extract in various concentrations (50, 100, 250 and 500 µg/mL) dissolved in buffer was added to the wells. After mixing, the plate was incubated for 15 min (at 25°C) and then the absorbance was measured at 412 nm in a microplate reader. The enzymatic reaction was initiated by the addition of 20 mL of 15 mM ATCI and the hydrolysis of acetylthiocholine was monitored by reading the absorbance every 5 min for 20 min. Berberine was used as a positive control. The percentage of inhibition of enzyme activity for each test solution was calculated using the following formula:
% inhibition of AChE activity = [(AB – AA) / AB] x 100
Where AA and AB denote the absorbance values of the test and the blank sample, respectively. The AChE inhibitory activities were examined and compared with berberine (positive control). The IC50 value was calculated from the % inhibition values of different concentrations of each plant extract.
Evaluation of neurotrophic activity
The neurotrophic activity of selected plant extracts was determined based on their capacity to promote neurite outgrowth in a primary culture of hippocampal neurons.
Culture of primary hippocampal neurons and extract treatment
All the reagents used for cell cultures were purchased from Invitrogen (Carlsbad, CA, USA) unless otherwise stated. The animal experiment was approved by the Institutional Animal Care and Use Committee of the Dongguk University College of Medicine (approval certificate number IACUC-2016-001). Time-pregnant rats (Sprague-Dawley) were ordered on the 13th day of pregnancy and housed at a controlled temperature with a light/dark cycle of 12/12 h and with access to food and water ad libitum. On the 19th day of pregnancy, the pregnant rat was euthanized with isofluorane and the fetuses were collected. The fetal hippocampi were then dissected from the brain and neuronal cultures were prepared as described previously [12]. Briefly, the dissected hippocampi were collected in Hank’s balanced salt solution (HBSS), and the tissues were dissociated by digestion with 0.25% trypsin in HBSS for 12 min at 37°C and trituration with fire-polished graded Pasteur pipettes. The dissociated cells were counted with a hemocytometer and plated at a density of 1.0 × 104 ~ 2.0 × 104 cells/cm2 onto poly-DL-lysine (PDL) (Sigma-Aldrich, St. Louis, MO, USA) -coated 12 mm glass coverslips in 24-wells culture plates. Cultures were maintained in a defined serum-free neurobasal media supplemented with B27 and incubated at 37oC under 5% CO2 and 95% air. Extracts or vehicle (DMSO, the final concentration of ≤0.375 %) was added to the media before cell plating. A normal control (media only) and vehicle control (media with DMSO) cultures were always compared with those treated with test samples.
Image acquisition, analysis and quantification
A Leica Research Microscope DM IRE2 equipped with I3 S, N2.1 S and Y5 filter systems (Leica Microsystems AG, Wetzlar, Germany) was used for phase-contrast microscopy. Images (1,388 × 1,039 pixels) were acquired with a high-resolution CoolSNAPTM CCD camera (Photometrics Inc., Germany) under the control of a computer using Leica FW4000 software. The digital images were processed using Adobe Photoshop 7.0 software.
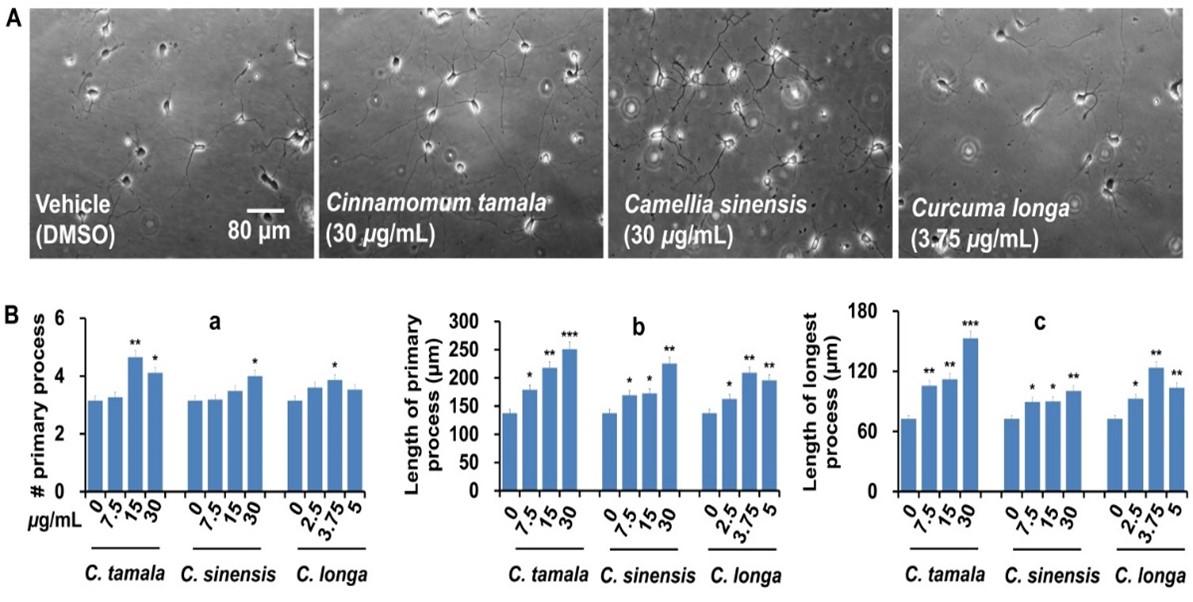
Morphometric analyses and quantification were performed with Image J (version 1.45) software with the simple neurite tracer plug-in (National Institute of Health, USA). Morphometric parameters such as the number of primary processes (neurites that originated directly from the soma), the total length of primary process (the sum of the length of primary neurites), and the length of the longest process were measured. Neurons (a minimum of 20 cells) that were not intermingled with the processes of adjacent neurons were selected for analysis. In this study, neurons of media only and vehicle (DMSO, up to 0.375%) controls exhibited very similar patterns of growth. Therefore, extract-treated cultures were always compared with vehicle control during morphometric analysis.
Statistical analysis
Data are expressed as mean ± standard deviation (with at least triplicate analysis). Data were analyzed using a one-way analysis of variance (ANOVA) with post hoc Duncan multiple comparisons, with a significant difference at p < 0.05 (SPSS software, version 16.0).
RESULTS
A total of 19 plant extracts were evaluated for their antioxidant, anticholinesterase, and neurotrophic activities. In this study, aqueous ethanol (95%) was used as an extraction solvent as most of the compounds, both polar and non-polar, are readily soluble in this solvent. The extract yields ranged from 4 to 20% (w/w) (Table 2).
Table 2. Extract yield and antioxidant activity of plant extracts.
Screening of medicinal plants for antioxidant activity
A total of 19 plant extracts with four different concentrations (0.01, 0.1, 0.5, 1.0 µg/mL) were evaluated for their antioxidant activity using DPPH radical scavenging assay. Of the extracts, C. sinensis exhibited the highest antioxidant activity with an IC50 value of 24.24 µg/mL, which was comparable to the positive control, vitamin C (IC50 value of 7.44 µg/mL) (Table 2). T. chebula, C. tamala, T. bellirica, and P. emblica also exhibited a significantly higher antioxidant activity with IC50 values of 38.54, 45.87, 56.84 and 62.12 µg/mL, respectively. C. longa showed a moderate antioxidant activity with an IC50 value of 107.28 µg/mL.
Anticholinesterase activity of medicinal plants
After initial screening, a total of six plant extracts showing promising antioxidant activities such as C. sinensis, T. chebula, P. emblica, T. bellirica, C. tamala, and C. longa were chosen to further evaluate their acetylcholinesterase inhibitory activity. As compared to positive control berberine (IC50 0.37 µg/mL), C. longa showed the lowest IC50 value (IC50 value of 9.3737 µg/mL) followed by C. sinensis (IC50 value of 86.0137 µg/mL) and C. tamala T. (IC50 value of 86.3737 µg/mL) (Table 3). Anticholinesterase activities of other extracts were insignificant.
Table 3. AChE inhibitory activity of plant extracts.
Neurotrophic activity of medicinal plants
We next evaluated neurite outgrowth promoting potentials of selected plant extracts that exhibited promising antioxidant and anticholinesterase activities. Morphological analysis revealed that plant extracts dose-dependently promoted neurite outgrowth in primary hippocampal neurons (Figure 1). As compared to control, neurons treated C. tamala (at 30 μg/ml) exhibited the highest increase in primary neurites (30%), length of longest neurite (110%), and the total length of primary neurites (82%) (Figure 1). Although the effects were moderate, C. sinensis and C. longa also significantly increased the indices of neurite outgrowth. These findings indicate that C. tamala, C. sinensis, and C. longa possess promising neurotrophic activity along with the antioxidant and anticholinesterase activities.
DISCUSSION
Here, an investigation on the neuropharmacological properties of indigenous medicinal herbs revealed that C. tamala, C. sinensis, and C. longa were among the herbs that showed significant antioxidant, anticholinesterase, and neurotrophic activities. These medicinal herbs could be valuable to explore novel neuroprotective agents that can target oxidative stress, cholinergic deficits, and neurodegeneration.
As oxidative stress plays an important role in the pathobiology of neurodegenerative disorders, pharmacological targeting of this pathogenic factor by antioxidants could intervene in the disease progression. From this perspective, traditional herbs that naturally contain potential antioxidant principles could be an attractive option. A remarkable antioxidant activity of C. sinensis (IC50 24.24 µg/mL), C. tamala (IC50 45.87 µg/mL), and C. longa (IC50 107.12 µg/mL), which is concomitant to the antioxidant-enriched traditional medicinal herbs [13], could play an important role in protecting against oxidative stress in brain disorders [14].
In the CNS, the cholinergic neurons innervate almost all regions of the brain [15]. According to the cholinergic hypothesis, a reduction in ACh level in the brain and specific degeneration of cholinergic neurons potentially contribute to AD pathology [16]. One of the potential therapeutic strategies is, therefore, to increase the ACh level in the brain by inhibiting AChE. AChE inhibitors that can increase the function of neural cells by increasing the concentration of ACh [17] are currently in use to treat AD patients. An ethnopharmacological survey [7] reports AChE inhibitory activity of traditionally used medicinal plants. In line with these, the present study also demonstrated that C. longa (IC50 9.37 µg/mL), C. sinensis (IC50 86.01 µg/mL), and C. tamala (IC50 86.37 µg/mL) exhibited promising anticholinesterase activity. These findings have also been supported by the previous other studies where similar anticholinesterase activities of C. longa [18] and C. tamala [19] were observed. Besides, when treated with 0.5% green tea (C. sinensis) for 8 weeks, a significant decrease in AChE activity was reported especially in cerebrum and cerebellum regions of the rat brain, ensuring a sufficient Ach level to sustain cholinergic neurotransmission [20].
The region-specific degeneration and atrophy of brain neurons along with the reduction of neurotrophic factors level is a common pathogenic feature of AD [4, 5]. The damaged neuronal networks can be reestablished by regaining the neuritogenic capacity which can be achieved through neuropharmacological modulators. Neurite outgrowth promoting activity of C. tamala, C.sinensis, and C. longa indicates that these traditional herbs could be promising exogenous sources of neurotrophic factor-like substance(s), supporting the integration of neuronal networks by inducing axonal and dendritic remodeling. A line of evidence also supports neuromodulatory and neuroprotective functions of C. longa and its main bioactive curcumin [18, 21-23] and C. sinensis [24].
The leaf extract of C. tamala (bay leaf) contains numerous polyphenolic compounds and essential oil which may underlie its antioxidant activity. In addition, the high anticholinesterase activity of C. tamala leaf extract in the present finding might be due to the presence of linalool, a lead bioactive component isolated from essential oils and leaf extract of C. tamala, showing a potent anticholinesterase activity in an in vitro study [19]. Green tea (C. sinensis) is another medicinal herb that possesses various pharmacological properties, including antioxidant [25] and neuroprotective [24] properties. C. sinensis is also rich in various phytochemicals including catechins, flavonols, flavanones, and phenolic acids [26]. Evidence [27-30] suggest that C. sinensis possesses high antioxidant activity which is in line with the current finding. In addition, due to the promising anticholinesterase activity, C. sinensis has the potential to enhance cholinergic function and therefore may have a role in ameliorating the cholinergic deficit in the AD and other age-related memory impairments [31]. Turmeric (C. longa) is a popular spice in the Indian subcontinent, including Bangladesh, that possesses natural polyphenolic alkaloids such as curcumin, desmethoxycurcumin, and bis-desmethoxy curcumin having potent antioxidant activity. Turmeric has a potent ability to inhibit the level of AChE and thereby positively influence neurotransmitter production, and also increase the dendritic arborization to enhance the learning and memory functions [18].
With these preliminary findings, this study provides a scientific rationale for the folk use of medicinal plants as a remedy for memory impairment and other neurological disorders. Plant-derived AChE inhibitors are comparatively safer than synthetic ones. Therefore, the current findings on the indigenous medicinal plants could provide some valuable information for the development of novel, clinically effective, and safe cholinesterase inhibitors, and neuroprotective agents to be used in neurological disorders. However, further bioassay-guided isolation and identification of active compound(s) responsible for the antioxidant, anticholinesterase, and neurotrophic activities are warranted. In addition, further evaluation is required to assess the safety and bioavailability of these herbs in animal models.
CONCLUSIONS
The present findings indicate that the indigenous medicinal herbs, particularly the leaves of Cinnamomum tamala and Camellia sinensis, and the rhizome of Curcuma longa exhibited a remarkable antioxidant, anticholinesterase, and neurotrophic potentials, suggesting that these popular herbs could be exploited as a potential source of neuroprotective compounds to be used in the development of disease-modifying therapies for central nervous system disorders.
ACKNOWLEDGEMENT
This research was co-funded by Bangladesh Agricultural University Research System (BAURES) to MAH (grant # 2015/38/AU-GC) and by the National Research Foundation of Korea (NRF) to Il Soo Moon (grant # NRF-2021R1A2C1008564). TA wishes to thank Ministry of Science and Technology, Government of The People’s Republic of Bangladesh for graduate assistance through NST fellowship.
AUTHOR CONTRIBUTIONS
MAH designed outlines and drafted the manuscript. TA, MAH, MNH, MM, and RD performed the experiments and analyzed the data. TA and MAH wrote the initial draft of the manuscript. MAH, AKMMI, MGM, MJU, ISM reviewed the scientific contents described in the manuscript. All authors read and approved the final submitted version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. Journal of Neurology, Neurosurgery & Psychiatry. 1999;66:137-47.
- [2]Hannan MA, Dash R, Sohag AAM, Haque MN, Moon IS. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. 2020;13.
- [3]Pandit MK. Neuroprotective properties of some Indian medicinal plants. Int J Pharm Biol Arch. 2011;2:1374-9.
- [4]Schaeffer EL, Novaes BA, da Silva ER, Skaf HD, Mendes-Neto ÁG. Strategies to promote differentiation of newborn neurons into mature functional cells in Alzheimer brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:1087-102.
- [5]Christensen DZ, Bayer TA, Wirths O. Intracellular Aβ triggers neuron loss in the cholinergic system of the APP/PS1KI mouse model of Alzheimer’s disease. Neurobiology of aging. 2010;31:1153-63.
- [6]Uddin MJ, Russo D, Rahman MM, Uddin SB, Halim MA, Zidorn C, et al. Anticholinesterase Activity of Eight Medicinal Plant Species: In Vitro and In Silico Studies in the Search for Therapeutic Agents against Alzheimer’s Disease. Evidence-Based Complementary and Alternative Medicine. 2021;2021:9995614.
- [7]Natarajan S, Shunmugiah KP, Kasi PD. Plants traditionally used in age-related brain disorders (dementia): an ethanopharmacological survey. Pharmaceutical Biology. 2013;51:492-523.
- [8]Rao RV, Descamps O, John V, Bredesen DE. Ayurvedic medicinal plants for Alzheimer’s disease: a review. Alzheimer’s research & therapy. 2012;4:1-9.
- [9]Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. Journal of ethnopharmacology. 2002;79:379-81.
- [10]Yen GC, Duh PD. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. Journal of agricultural and food chemistry. 1994;42:629-32.
- [11]Ellman GL. A colorimetric method for determining low concentrations of mercaptans. Archives of biochemistry and Biophysics. 1958;74:443-50.
- [12]Hannan MA, Kang JY, Hong YK, Lee H, Choi JS, Choi IS, et al. The marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytotherapy Research. 2013;27:21-9.
- [13]Shukla SD, Bhatnagar M, Khurana S. Critical evaluation of ayurvedic plants for stimulating intrinsic antioxidant response. Frontiers in neuroscience. 2012;6:112.
- [14]Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radical Biology and Medicine. 2002;33:182-91.
- [15]Woolf NJ, Butcher LL. Cholinergic systems mediate action from movement to higher consciousness. Behavioural brain research. 2011;221:488-98.
- [16]Bartus RT, Dean Rr, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408-14.
- [17]Tabet N. Acetylcholinesterase inhibitors for Alzheimer’s disease: anti-inflammatories in acetylcholine clothing! Age and ageing. 2006;35:336-8.
- [18]Devi N, Mukkadan J. Impact of rotatory vestibular stimulation and Curcuma longa on spatial learning and memory in Wistar albino rats. Asian J Pharm Clin Res. 2016;9:167-73.
- [19]Dalai MK, Bhadra S, Chaudhary SK, Bandyopadhyay A, Mukherjee PK. Anti-cholinesterase potential of cinnamomum tamala (Buch.-Ham.) T. nees & eberm. leaves. Indian Journal of Traditional Knowledge. 2014;13:691–7.
- [20]Kaur T, Pathak C, Pandhi P, Khanduja K. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain and cognition. 2008;67:25-30.
- [21]Kulkarni S, Akula KK, Deshpande J. Evaluation of antidepressant-like activity of novel water-soluble curcumin formulations and St. John’s wort in behavioral paradigms of despair. Pharmacology. 2012;89:83-90.
- [22]Hannan MA, Dash R, Sohag AAM, Haque MN, Moon IS. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Frontiers in molecular neuroscience. 2020;13:116.
- [23]Yang J, Song S, Li J, Liang T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathology-Research and Practice. 2014;210:357-62.
- [24]Lee S-R, Suh S-I, Kim S-P. Protective effects of the green tea polyphenol (−)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neuroscience letters. 2000;287:191-4.
- [25]Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen Z-Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. The Journal of nutrition. 2001;131:2248-51.
- [26]Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chemical Engineering and Processing: Process Intensification. 2003;42:129-33.
- [27]Chan EWC, Lim YY, Chew YL. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chemistry. 2007;102:1214-22.
- [28]Kerio LC, Wachira FN, Wanyoko JK, Rotich MK. Total polyphenols, catechin profiles and antioxidant activity of tea products from purple leaf coloured tea cultivars. Food Chemistry. 2013;136:1405-13.
- [29]Macedo JA, Battestin V, Ribeiro ML, Macedo GA. Increasing the antioxidant power of tea extracts by biotransformation of polyphenols. Food Chemistry. 2011;126:491-7.
- [30]Namal Senanayake SPJ. Green tea extract: Chemistry, antioxidant properties and food applications – A review. Journal of Functional Foods. 2013;5:1529-41.
- [31]Okello EJ, Savelev SU, Perry EK. In vitro anti‐β‐secretase and dual anti‐cholinesterase activities of Camellia sinensis L.(tea) relevant to treatment of dementia. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2004;18:624-7.